
Chiseled in gray granite over the entrance to the New York City Post Office on 8th Avenue are the words, “Neither snow nor rain nor heat nor gloom of night stays these couriers from the swift completion of their appointed rounds.” This unofficial motto for the United States Postal Service truly symbolizes the importance of high-fidelity delivery systems. For the clinical laboratory, timeliness and deliberate care in the delivery of patient samples are similarly essential to providing accurate test results for patient care.
Clinical samples may originate from catchment areas hundreds of kilometers away to within the same walls of the healthcare institution. A fundamental understanding of external and internal sample transport can equip the modern laboratorian for mitigating preanalytical error in this important area of the total testing process.
External Sample Transport
Worldwide, healthcare institutions continue to expand their outreach programs. As a result, higher volumes of laboratory samples routinely travel from offsite clinics to centralized, core clinical laboratories for testing. External sample transport involves many individual steps before samples reach the laboratory for analysis (Figure 1). Along the way, a sample’s journey passes through two critical phases, beginning with staff at the offsite physician office or outpatient clinic collecting the patient sample, processing it, and placing it inside or outside of the clinic for courier pickup, depending on the facilities’ hours of operation. After a clinic closes, samples are routinely placed inside an outdoor courier lockbox for off-hours access.
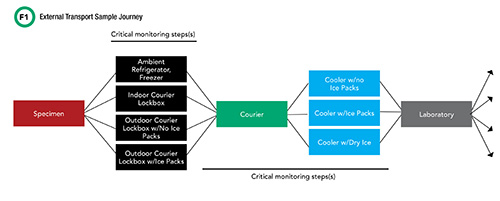
See Figure 1 in CLN June PDF
The second critical phase begins with the courier picking up the sample, and placing it in one of various cooler caddies within their transport vehicle, contingent on sample storage requirements. This is the longest part of the sample’s journey. Couriers often travel vast distances in their routes and may visit multiple pickup sites before finally reaching the destination clinical laboratory. Samples that reach this destination are then sorted into two categories—to be analyzed onsite or to be shipped using similar transport steps to an offsite reference laboratory.
Climate Zones
Climate zones are a major determinate of the protections biological samples will require for appropriate external sample transport. According to the International Energy Conservation Code (IECC), funded by the U.S. Department of Energy, Office of Energy Efficiency and Renewable Energy, the United States consists of several distinct climate zones (Figure 2). Each zone presents unique challenges for laboratorians to consider when optimizing their institution’s sample transport roadmap.
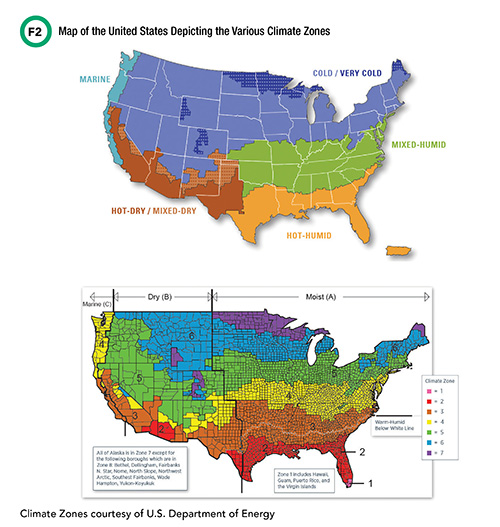
See Figure 2 in CLN June PDF
For example, on June 20, 2017, Phoenix, Arizona (hot-dry zone) recorded ambient air temperatures just under 50ºC, which grounded flights within the region. At the other extreme, on January 28, 2019, Minnesota (cold-very cold zone) experienced its coldest ambient air temperature (-56ºC) in more than two decades—the Arctic polar vortex of 2019. A reference lab within this region notified institutions across the U.S. that samples should not be shipped to its facility during this time and posted an alert on their website test catalog. Although these cases represent extremes, the laboratory service needs to consider both the average and extremes to develop a robust external sample transport program that minimizes preanalytical variables.
Courier Lockbox
Courier lockboxes are an often-overlooked step in the preanalytical phase of laboratory testing (1). These insulated lockboxes come in various shapes and sizes to accommodate different clinical sample types and volumes and are commonly made of thin, high-impact polystyrene or 20–24 gauge cold-rolled steel. For outdoor courier lockboxes, there are no universally acceptable standards or guidelines that provide recommendations on 1) sample storage time limits; 2) lockbox material and insulation; 3) lockbox location; or 4) maintenance of lockbox temperatures during seasonal extremes. This absence of guidelines is highlighted in a recent study that shows instructions for lockboxes are inconsistent between a selection of academic, private, and reference labs (1).
Based on our experience and recent publications, samples inside outdoor lockboxes exposed to ambient seasonal temperatures are a significance source of preanalytic error. Recent studies demonstrate that centrifuged and uncentrifuged lithium heparin samples stored in outdoor lockboxes with and without ice packs showed significant changes (1). These changes were apparent within 1 hour in summer temperatures in Charlottesville, Virginia. Real-time, continuous temperature monitoring revealed that lockboxes with ice packs had a mean temperature of 22.3ºC (range: 16.5–22.3ºC) whereas lockboxes without ice packs had a mean temperature of 42.6ºC (range: 34.4–46.9ºC).
In a similar study, outdoor courier lockboxes were monitored during four seasons in the southern region of the U.S. Inside steel, insulated lockboxes with no ice packs, the ambient outdoor temperatures demonstrated wide fluctuations over three days in spring (range: 7.0-25.2ºC) and fall (range: 2.2-22.8ºC) (2).
Courier Transport
Planes, trains, and automobiles are the most common transport vehicles used in external sample transport. Clinical laboratorians must fully understand each to optimize acceptable transport conditions. For example, samples boarding and deboarding planes encounter potentially unique conditions. Pallets of samples sitting on a tarmac waiting to be loaded onto or offloaded from a plane can be exposed to the extreme heat that is absorbed by dark asphalt. Similarly, samples being transported by train or automobile may be affected by exposure to sun through windows, air conditioners, heaters, and seasonal ambient temperatures. In addition, storage containers in any of these steps can be specific to institution or courier service, which introduces another level of variability.
Agitation or shock forces are an underappreciated problem and ripe for monitoring in external sample transport. Agitation can mix blood cells, cellular debris, fibrin, and potentially other sample contents that are separated or partially separated during the centrifugation process. One recent study—performed in association with a reagent formulation change—showed that samples analyzed directly after courier transport yielded falsely increased vitamin D concentrations (3).
To demonstrate that courier transport and agitation were the culprit, hundreds of samples in question were held upright overnight and retested. The same specimens were then intentionally mixed and reanalyzed. After deliberate inversion, samples showed comparable results to those being analyzed directly after courier transport, whereas results generated when held overnight decreased (3).
Integrated External Sample
Transport System
Integrated sample transport systems are a possible solution to for standardizing external sample transportation. Researchers in Padova, Italy, used a system with secondary and tertiary transport containers that could be monitored and tracked in various stages of delivery. Over several years of their study, the authors were able to establish standard operating procedures and characterize courier routes associated with their offsite collection centers.
Through these efforts, they increased the number of overall courier trips with acceptable temperatures and reduced both unacceptable temperatures and excessive transport times defined institutionally as < 20°C, >25°C, and >3 h, respectively (4). This group also demonstrated the feasibility of a state-of-the-art, temperature-controlled delivery system, akin to a small refrigerator (5).
Internal Sample Transport
Pneumatic tube systems are an integral feature of most healthcare institutions and enable safe and efficient transport of clinical samples for centralized testing. A schematic drawing of a basic pneumatic tube system is depicted in Figure 3. These systems use a network of pipelines to transport capsules, also known as carriers, from one point to another.
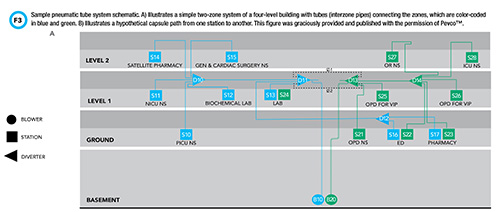
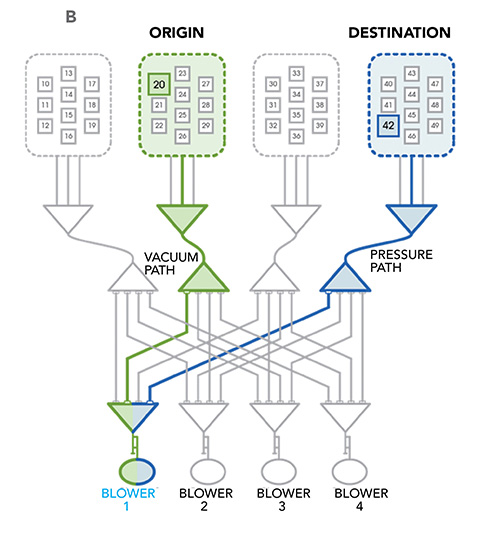
See Figure 3 in CLN June PDF
Capsules are sent and received at ports known as stations. Stations are strategically located throughout the healthcare institution to facilitate transport between sites with the highest sample traffic. Often, capsules do not travel a straight path from one station to another; in fact, pneumatic tube systems are frequently complex and branched. An apparatus known as a diverter enables carriers to change direction. Diverters can be likened to railroad switches that enable trains to switch from one track to another.
What drives the capsules through this network of tubes? As the name “pneumatic” suggests, a blower generates changes in air pressure that move capsules through the system. In contrast to inert materials, clinical samples are sensitive to changes in air pressure and shock forces (e.g., 3-dimensional or 3-axis acceleration) experienced inside the system. The effect of these forces on sample integrity and the accuracy of downstream analyses is well documented. Pneumatic tube transport can introduce bias in the measurement of several chemistry analytes, blood gas measurements, and hemostasis and platelet studies. In the arena of blood banking, pneumatic tube transport has deleterious effects on the integrity of blood products.
To date, no clinical laboratory regulatory body has outlined requirements to measure or mitigate the impact of pneumatic tube transport on the accuracy of downstream testing. A limited discussion of the effect of pneumatic tube transport on blood gas measurements and specimen hemolysis follows below and is meant to provide a framework for some of the considerations around this issue.
Can Blood Gas Specimens Be Tubed?
In the clinical laboratory, one of the most widely studied challenges associated with pneumatic tube systems is the transport of samples destined for blood gas analyses. For example, in blood samples with a partial pressure of oxygen (pO2) lower than atmospheric pO2 (approximately 159 mmHg at sea level), oxygen from air bubbles can diffuse into the sample resulting in falsely increased oxygen, while oxygen can diffuse from hyper-oxygenated samples into air bubbles.
Most blood gas analyzer manufacturers recommend purging air from samples and capping syringes with a rubber stopper to minimize air contamination. Furthermore, the College of American Pathologists requires laboratories to implement procedures to prevent ambient air contamination of samples for blood gas testing and document suspected erroneous results. However, the vigorous shaking that can occur as specimens are whisked through the pneumatic tube system can generate air bubbles and enhance diffusion between air bubbles and the sample.
Positive and negative air pressure changes that occur as capsules travel through the tube system may also affect blood gas analytes. Several studies have investigated this issue with varying conclusions as to whether pneumatic tube transport introduces clinically significant biases in blood gas results. This variability likely reflects differences in tube system characteristics, including distance traveled by the sample, route, number of diversions, air pressure, and magnitude and number of accelerations (6).
One study observed a mean bias of 8.0 mmHg in pO2 in arterial blood gas samples that were tubed compared to those walked to the laboratory for analysis but determined that this difference was neither clinically nor statistically significant (7). A mean bias of 0.94 mmHg was observed for partial pressure of carbon dioxide (pCO2) in arterial blood gas samples in the same study.
In contrast, another study found a statistically significant mean bias of 3.16 kPa (approximately 24 mmHg) in arterial pO2 between specimens analyzed at the point of care compared to those transported via pneumatic tube system for analysis (8). Transport of samples in pressure-sealed containers resulted in a reduced mean bias that was determined to be statistically insignificant. Interestingly, there was no correlation between transport time, which ranged from approximately 7 to 42 minutes, and the magnitude of the differences observed (7).
These contrasting studies are two of many that support the need for clinical laboratories to empirically determine the effect of pneumatic tube system transport on specimens destined for blood gas measurements.
Air Bubbles Caused By Pneumatic
Tube Transport Induce Hemolysis
Another effect of pneumatic tube transport on blood samples is the induction of hemolysis. Mullins et al. measured the magnitude and temporal pattern of 3-axis acceleration by sending a bubble-wrapped smartphone with kinetic sensing applications through the pneumatic tube system (6). The experiment showed that the magnitude of forces experienced by tubed samples was at least 8 x g in contrast with hand delivered samples, which did not experience forces in excess of 2 x g. Samples transported through routes with longer transport times and larger acceleration forces had more hemolysis—as measured by hemolysis index and increased concentrations of lactate dehydrogenase (LDH)—compared to those transported by hand or through a shorter route with smaller acceleration forces. Number of forces experienced positively correlated with the degree of hemolysis and LDH elevation.
This study also hinted that air bubble formation during transport may contribute to sample hemolysis through hydrodynamic stress. In a follow-up study (9), the same group showed that artificial generation of bubbles in samples using a gentle air stream induced hemolysis, with accompanying increases in LDH and potassium.
Importantly, this study showed that the production of air bubbles in a specimen is sufficient to induce hemolysis and lead to false changes in analytes affected by hemolysis, even in the absence of acceleration forces. Hemolysis was reduced by complete filling of blood collection tubes to minimize air space present in the tubes (and consequently, the potential for bubble formation), further supporting the relationship between air bubble induction and sample hemolysis.
Validation of Hospital
Pneumatic Tube Systems
Given the variability of effects on blood samples between and even within hospitals, it has become increasingly evident that each institution should validate its pneumatic tube system for blood transport. Farnsworth et al. outlined parameters for such a validation, including the need to examine different routes, measure intraday and interday variability (elapsed time, area under the curve, number and magnitude of accelerations), and determine whether transport induces clinically significant changes in analytes (10).
Another study focused largely on comparing four commercially available 3-axis accelerometers for use in monitoring hospital pneumatic tube systems (11). This study demonstrated differences in performance between accelerometers with respect to the maximum force the devices were capable of measuring and the failure rate of the devices. There are currently no regulatory requirements to validate the performance of pneumatic tube systems, and harmonization and minimum performance characteristics of 3-axis accelerometers are also lacking. Given these limitations, the authors of the study recommend using a single device for all validation studies.
Quality Management Recommendations for Sample Transport
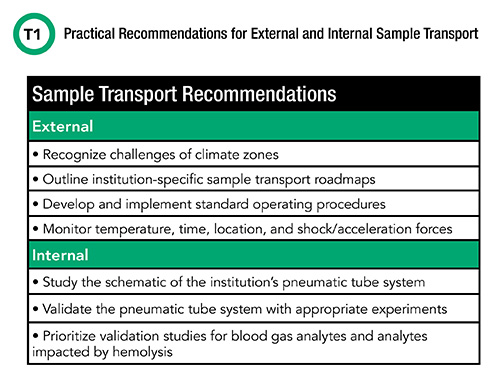
With the largest proportion of errors in clinical laboratory testing attributed to the preanalytical phase, mitigating the negative impacts of sample transport represents an opportunity to improve the quality and accuracy of laboratory testing. The first, and perhaps most important, step of implementing meaningful interventions is the ability to identify and quantitate the effects of various transport modalities. Table 1 outlines several recommendations for the assessment and measurement of the effects of both external and internal transport on samples. Once identified, laboratory leaders can implement appropriate mitigation strategies and monitor them for effectiveness.
References
- Dibbern ME, Pierre CC, Wiencek J. Outdoor courier lockboxes in summer are a significant source of preanalytical error. (In press.)
- Wiencek J, Nichols JH. Impact of ambient seasonal temperature on specimens stored in courier lock-boxes. J Appl Lab Med 2018;2:973–6.
- Hernandez JA, Stanford JE, Savoie CV, et al. Spuriously elevated 25-Hydroxyvitamin D in lithium heparin plasma samples transported by courier. J Appl Lab Med 2018;2:809–811.
- Zaninotto M, Tasinato A, Padoan A, et al. An integrated system for monitoring the quality of sample transportation. Clin Biochem 2012;45:688–90.
- Zaninotto M, Tasinato A, Vecchiato G, et al. Performance specifications in extra-analuytical phase of laboratory testing: Sample handling and transportation. Clin Biochem 2017;50:574–8.
- Mullins GR, Harrison JH, Bruns DE. Smartphone monitoring of pneumatic tube system-induced sample hemolysis. Clin Chim Acta 2016: 462:1–5.
- Carabini LM, Nuriel J, Diaz Milan R, et al. The clinical significance of patient specimen transport modality: Pneumatic tube system impact on blood gas analytes. Respir Care 2016:61:1311–1315
- Collinson PO, John CM, Gaze DC, et al. Changes in blood gas samples produced by a pneumatic tube system. J Clin Pathol 2002;55:105–7.
- Mullins GR, Bruns DE. Air bubbles and hemolysis of blood samples during transport by pneumatic tube systems. Clin Chim Acta 2017: 473:9–13.
- Farnsworth CW, Webber DM, Krekeler JA, et al. Parameters for validating a hospital pneumatic tube system. Clin Chem 2019:65:694–702.
- Franks CE, Krekeler JA, Gronowski, et al. A comparison of four 3-axis-accelerometers for monitoring hospital pneumatic tube systems. J Appl Lab Med 2020:5:1345–50.
Christina C. Pierre, PhD, DABCC, is a clinical chemist at Penn Medicine Lancaster General Hospital in Lancaster, Pennsylvania. + E-mail: [email protected]
Joe Wiencek, PhD, DABCC, FADLM, is an assistant professor at Vanderbilt University School of Medicine and medical director of the core clinical chemistry laboratory at Vanderbilt University Medical Center in Nashville, Tennessee. +Email: [email protected]