Patient-centered care has become a popular term within the medical establishment. The commonly accepted definition provided by the Institute of Medicine is: “Providing care that is respectful of, and responsive to, individual patient preferences, needs and values, and ensuring that patient values guide all clinical decisions” (1).
From an ethical perspective, being patient-centered means respecting individuals’ autonomy and values in all that we do. It means reimagining our systems and processes through patients’ eyes and being willing to give up both convenience and control on the provider side when necessary. From a practical perspective, patient-centered care can improve the effectiveness of care by breaking down barriers to patient engagement and access.
In this article, we apply some of these patient-centered principles to laboratory stewardship, with a special focus on access to testing.
Patient-centered Stewardship
Given that healthcare organizations, including laboratories, are generally operated as businesses, any discussion of patient-centeredness must also address the relationship between healthcare corporations and the patients they serve. Just prior to the pandemic, one of us proposed the concept of “patient-centered capitalism,” whereby the interests of patients would be explicitly prioritized over those of financial and other stakeholders within both the healthcare system and its suppliers. The idea, summarized, is that “…healthcare will find it easier than many other industries to find the right balance among its various groups. The doctrine of shareholder supremacy has a certain elegance in that it simplifies the decision about which group comes first: shareholders. Medical ethics and patient-centered capitalism offer an equally elegant solution: patients come first and all others, including shareholders, come second” (2).
Stewardship refers to the careful management of an essential resource. Traditionally, lab stewardship is defined as a set of activities, policies, and procedures that improve four elements of lab testing: test ordering, result retrieval, result interpretation, and financial fairness for patients and other stakeholders (3).
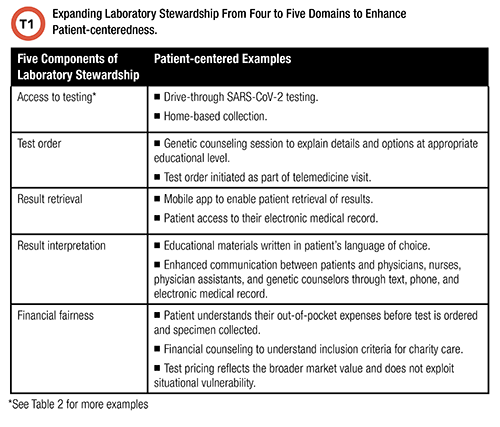
It stands that for laboratory stewardship to be patient-centered, the scope must include access on the part of the patient because the correct test is irrelevant if the patient is unable to complete the testing process.
Patient-centered Innovation
Table 2 lists examples of expanded physical access to testing. These include home testing, self-collection at home, mobile collection by healthcare professionals, and increased community collection and testing sites. These services were available, and slowly improving, before the pandemic, but the pandemic dramatically expanded them as a logical response to an international emergency. The economics of these services—for example, through reasonable reimbursement for SARS-CoV-2 testing—became more favorable during the pandemic, and this fueled innovation.
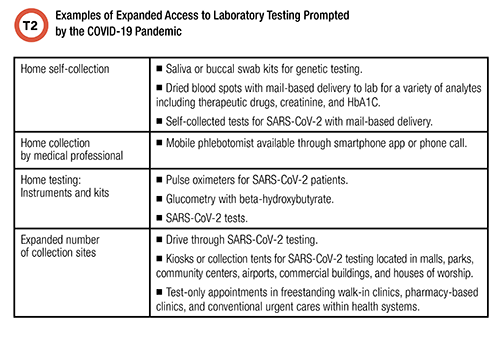
Patients throughout the United States clearly like the convenience that the pandemic necessitated. Health systems have responded by integrating improved access to lab testing into their scope of care, which now includes a significant telemedicine component. It follows that expanded access to testing is likely to persist, and innovation will accelerate.
Self-collection is enabled by technologies that make it easier to collect and properly label any specimen, including dried blood spots, capillary blood, saliva, nasal or nasopharyngeal swabs, buccal swabs, urine, and stool. For example, the use of dried blood spots, ordered through telemedicine and self-collected at home, is feasible for chronic disease monitoring, and this usage expanded during the pandemic (4). Similarly, access to genetic testing has improved due to expanded use of saliva and buccal sample collection kits through home collection.
Mobile collection is enabled by a variety of technologies, and it is best if these technologies are redundant. For example, in some metropolitan areas, patients with smartphones can now order mobile phlebotomy services from an app that is analogous to Door Dash or Uber, and those without a smartphone can just call a phone number to request the service. Northwell Health, which launched an app-driven mobile phlebotomy service called LabFly in August 2019, scaled that service to more than 500 patients per day during the pandemic (5).
Self-collection, mobile collection, and alternative-site collection are not only more convenient for the patient, but they also have distinct clinical and public health advantages. During the pandemic, alternative collection sites have helped reduce infectious exposures for both healthcare workers and patients. They also have led to patients being tested who otherwise might not have been tested, a benefit that could apply to other tests as well.
Expanded Testing at Home
Home testing is most patient-centered when done in collaboration with care providers. This is particularly important for monitoring chronic conditions. If a broader menu of safe point-of-care instruments and collection devices were available at home, chronically ill patients could partner with their providers to establish a monitoring plan for a variety of useful testing such as therapeutic drug monitoring, markers of disease burden (e.g. viral loads), and biomarkers of organ function and damage. Patient-centered stewardship is enabled by manufacturers that develop reliable, broad-menu instruments that surpass regulatory requirements and are usable by a wide range of patients, while connecting to the patient’s care providers and medical records.
Avoiding Excesses—Including Quackery
Improved access to testing is necessary but not sufficient to enhance patient-centered laboratory stewardship. Improved access only enables stewardship if the testing is medically necessary.
Removing barriers to access likely will expand the waste and abuse that have long plagued the laboratory industry (6). These include: overbundling of tests, quackery, and releasing tests for clinical use before there is reasonable, independent evidence of their clinical utility. Lack of evidence for clinical utility is particularly problematic regarding tests marketed directly to patients. For example, direct-to-consumer genetic testing aimed at guiding a patient’s diet or exercise remains largely unproven in producing better outcomes.
Laboratory stewardship programs that put in safeguards to avoid these excesses, while expanding access to medically necessary testing, will be on the vanguard of improving patient-centered care.
References
- Institute of Medicine. Crossing the quality chasm: A new health system for the 21st century. Washington DC: National Academy Press 2001.
- Jackson BR. Patient-centered capitalism: Redefining ethics in the medical industry.http://www.statnews.com/2019/09/24/patient-centered-capitalism-redefine-ethics-health-care. (Accessed May 6, 2021).
- Dickerson J, Fletcher AH, Procop G, et al. Transforming laboratory utilization review into laboratory stewardship: Guidelines by the PLUGS National Committee for Laboratory Stewardship. JALM 2017;2:259-68.
- Roberts AJ, Malik F, Pihoker C, et al. Adapting to telemedicine in the COVID-19 era: Feasibility of dried blood spot testing for hemoglobin A1c. Diabetes Metab Syndr 2021;15:433-7.
- Jackson BR. Bringing phlebotomy services to the home and workplace with LabFly. https://arup.utah.edu/education/podcasts/labmindEp37.php (Accessed May 6, 2021).
- Astion ML. How to say no to sendouts: Strategies for coping with tests that aren't ready for clinical use. http://www.myadlm.org/cln/articles/2019/october/how-to-say-no-to-sendouts. (Accessed May 6, 2021).
Michael Astion, MD, PhD, is medical director of the department of laboratories at Seattle Children’s Hospital and a clinical professor in the department of laboratory medicine and pathology at the University of Washington. +Email: [email protected]
Brian R. Jackson, MD, is an associate professor at the University of Utah School of Medicine and medical director of support services, IT, and business development for ARUP Laboratories. +Email: [email protected]