
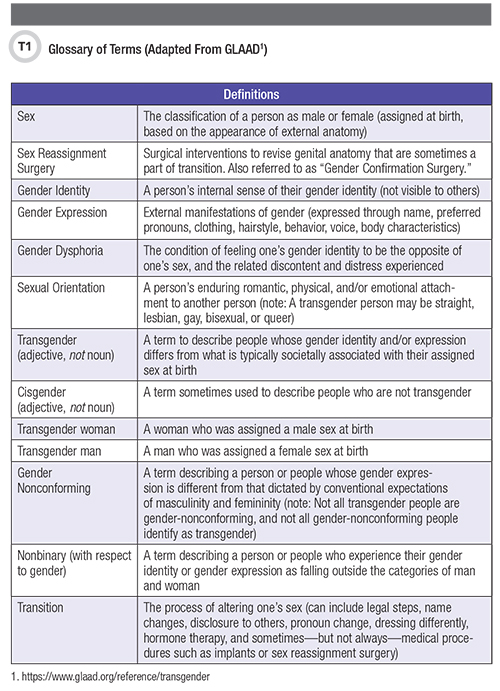
Transgender, or trans, people experience a discordance between their gender identity and the sex they were assigned at birth (1). Worldwide, transgender individuals represent a diverse group with diverse concerns. Prevalence estimates are flawed by varying definitions and incomplete, inaccurate reporting, but suggest that 0.3% to 0.5% of people identify as transgender (2). For context, Table 1 summarizes common terms as they relate to sex, gender, and sexual orientation.
Trans people bear a disproportionate burden of mental health distress, substance use disorders, and sexually transmitted infections across low-, middle-, and high-income settings, as well as a disproportionate burden of suicide (3, 4). Further exacerbating these health inequities, they often have difficulty accessing care, both for their gender-related needs and more routine medical care (2, 5). Notably, transgender women have a 19.1% prevalence of HIV and 49-fold higher odds of acquiring HIV in their lifetimes than all adults of reproductive age globally (6).
This review focuses on laboratory testing of transgender people as part of clinical care, as well as possible interactions between hormone therapies (testosterone and estradiol) and relevant pharmacotherapies, with a focus on pre-exposure prophylaxis (PrEP) and antiretroviral therapies (ART). Optimal drug therapy for any patient involves careful consideration of the complex interplay between hormones (both exogenous and endogenous) and drugs.
Understanding Gender-Affirming Therapies
A substantial subset of trans people use gender-affirming therapies, which often include various formulations of exogenous hormones like testosterone and estrogen, as well as androgen blockers. Table 2 (available online at aacc.org/publications/cln) depicts common components of gender-affirming hormone therapy (GAHT) regimens.
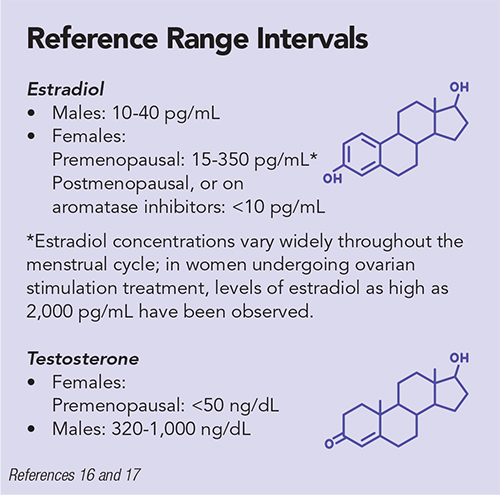
Typically, GAHT regimens are adjusted to achieve testosterone concentrations <50 ng/dL and estradiol concentrations of 100-200 pg/mL for transgender women and testosterone levels of 300-1,000 ng/dL for transgender men (1).
However, these benchmarks are only a guide for open discussion between providers and patients: The goal of treatment is to reduce gender dysphoria, rather than to precisely target a certain laboratory value. The World Professional Association for Transgender Health publishes and periodically updates a useful resource for standards of clinical care and laboratory result interpretation for transgender people (1).
Guidelines recommend maintaining physiologic levels of gender-appropriate hormones as well as surveilling for known complications associated with hormone therapy. The risks of uncontrolled and unmonitored GAHT include, but are not limited to, cardiovascular disease, venous thromboembolism, cancers, prolactinoma, and osteoporosis.
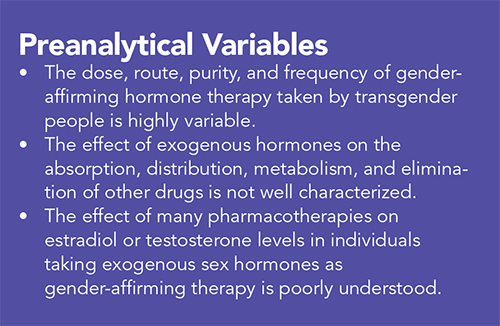
Transgender people require just as broad an array of pharmacotherapies as non-transgender individuals. Due to the lack of access to appropriate or consistent healthcare, and in conjunction with medical mistrust that exists within the community, trans people might use the internet or black market to acquire pharmaceutics and self-prescribe GAHT and other drugs such as nonsteroidal anti-inflammatory drugs and antibiotics (7–9). Self-prescribing and self-injection with silicone and other “fillers” might affect both the efficacy and the toxicity of GAHT and other pharmacotherapies.
Numerous sex-based differences in factors determine pharmacokinetics and pharmacodynamics, which may or might not apply to the situation of GAHT-induced differences in those parameters. These factors, including the impact of endogenous and exogenous sex hormones on pharmacokinetic parameters, have not been clearly elucidated.
Sex-dependent differences in the expression of hepatic metabolizing enzymes have been described in animals, and human studies show a trend toward increased metabolism of CYP 3A4 substrates in women as compared to men (13, 14).
Other factors impacting pharmacokinetics—and thus, drug activity—that can vary by sex include: gastric emptying time (bioavailability); volume of distribution (based on a higher percentage of body fat in women); renal excretion; protein binding of drug in plasma and tissue; and blood flow to organs (15).
Notably, the laboratory reference ranges specified here and elsewhere depend critically on both assay methodology and the particular platform used to measure hormone levels. There will be variability based on both of these factors, among others.
Hormone Testing
Generally, estradiol as measured by immunoassay has a limit of quantitation of 25 pg/mL; however, some assays have lower limits of quantitation (16). Because immunoassays tend to demonstrate poorer analytical performance at very low estradiol concentrations (<25 pg/mL), liquid chromatography-tandem mass spectrometry (LC-MS/MS) is the preferred method for the assessment of estradiol concentrations in children, males, and postmenopausal females.
Further, depending on how recently they’ve received exogenous hormone therapy, this may also be the case for transgender women in certain scenarios (18). However, because 70% of these women are on GAHT, LC-MS/MS typically is not recommended and immunoassay-based estradiol measurements are sufficient. Their care can also include monitoring testosterone concentrations in addition to estradiol concentrations.
Most studies between drugs and hormone therapy focus on contraceptive agents such as ethinyl estradiol, which are a different form of estrogen than the 17β-estradiol prescribed in GAHT. While some transgender women use contraceptive agents such as medroxyprogesterone acetate (Depo-Provera) and conjugated estrogens (Premarin), the latter are no longer recommended due to a potential for thrombogenicity and difficulty measuring blood levels.
In addition, the doses given in GAHT are generally much higher than those given for menopausal symptoms. For example, a typical dose for hormone replacement therapy is 0.02 mg oral ethinyl estradiol daily, 2 mg of oral 17β-estradiol daily (or 0.05 mg transdermal estradiol patch twice weekly), while a typical dose for transgender women is 5–20 mg intramuscular estradiol valerate (or 0.1–0.4 mg transdermal estradiol patch twice weekly).
While estradiol levels are useful to monitor oral, intramuscular, or transdermal preparations, they cannot be used to monitor synthetic or conjugated equine estrogens (which are in any event not recommended for use as GAHT, because of high risk of venous thromboembolism) (11).
Laboratories can measure testosterone in serum via immunoassay or more sensitive isotope dilution (ID)-LC-MS/MS methods (19–22). Free testosterone is that portion of testosterone in serum which is not bound to serum proteins (generally about 2%–3% of total), as described by the equation: Total testosterone = Free testosterone + Albumin-bound testosterone + Sex hormone binding globulin (SHBG) bound testosterone (23).
To determine the amount of bioavailable testosterone (free testosterone + weakly albumin-bound testosterone) present, a simple calculator integrates albumin, testosterone, and SHBG concentrations (http://www.issam.ch/freetesto.htm).
Laboratories might also measure both free testosterone and total testosterone. The latter is most commonly measured in transgender women and transgender men, consistent with the recommendations of the Endocrine Society, as free testosterone assays are less reliable (11). The immunoassay performs particularly poorly at low serum testosterone levels, such as those often seen in cisgender women. In general, for injectable forms of testosterone supplementation in transgender men, the timing of testosterone levels should be midway between injections.
Pharmacotherapies
Metabolism of Sex Hormones
Estradiol is metabolized to estrone at the intestinal lining by 17β-hydroxysteroid dehydrogenase, and undergoes first-pass metabolism to a broad array of metabolites in the liver (24). Testosterone is metabolized mainly in the liver by conjugation or converted to 17-ketosteroids by 5α- and 5β-reductases among other enzymes. Delivery strategies other than oral (intramuscular or transdermal) avoid the first-pass effect and result in more stable levels. Pharmacotherapies that impact the CYP system would not be expected to affect estradiol metabolism or concentrations.
Antiretrovirals and Preexposure Prophylaxis
The last 30 years have seen a revolution in the treatment and prevention of HIV; there are now more than 25 approved antiretrovirals in six different classes, and administering certain antiretrovirals prior to HIV exposure has been proven to have high (up to 99%) efficacy in preventing HIV (25–31).
Emtricitabine 200 mg/Tenofovir disoproxil fumarate 300mg (F/TDF)
The iPrEx study showed 44% protective efficacy of oral Truvada (F/TDF) versus placebo in 2,499 men who have sex with men (MSM) and transgender women; among those with tenofovir-diphosphate (TFV-DP) concentrations commensurate with 4 or more doses per week, the protective efficacy was 96% (27, 31). In a subgroup analysis of the 339 transgender women included in the study, seroconversion events were the same in groups randomized to F/TDF and placebo. However, upon pharmacologic analysis of the seroconverters, none of those randomized to the F/TDF arm had detectable drug concentrations. These data suggest that the seroconversions were caused by a lack of drug adherence, rather than an inefficacy of drug in this subgroup of participants; transgender women who remained HIV negative maintained detectable drug concentrations during study visits. Furthermore, transgender women who reported condomless receptive anal intercourse (cRAI) were less likely to have detectable drug concentrations than were MSM who reported cRAI (32).
In a subgroup analysis, seroconversion events did not differ between trans people randomized to TDF/FTC or placebo. However, upon pharmacologic analysis, none of the seroconverters randomized to the TDF/FTC arm had detectable drug concentrations. These data suggest a lack of drug adherence as a cause for seroconversion; transgender women who remained HIV negative maintained detectable drug concentrations during study visits (20). Further, drug detection was lower among transgender women who reported cRAI than among MSM who reported cRAI.
Recent studies have examined potential interactions between feminizing GAHT and F/TDF. No studies on the interaction between PrEP and masculinizing GAHT or testosterone concentrations have yet been published, though the ongoing I-BrEATHe study (a pharmacokinetic substudy of the larger TRIUMPH PrEP demonstration project in transgender communities) will assess drug-drug interactions between F/TDF and GAHT in both transgender women and transgender men. The iFact study in transgender women in Thailand found that co-administration of F/TDF did not affect estradiol concentrations, but that estradiol lowered plasma tenofovir (TFV) concentrations by 12%; although the dosing of PrEP was not directly observed, several adherence measures were in place (33).
A second study in eight transgender women on GAHT (with estradiol concentrations >100 pg/mL) and eight cisgender men (CGM) in Baltimore examined the effect of GAHT on concentrations of TFV, FTC, and their active metabolites, in plasma as well as intracellularly within peripheral blood mononuclear cells and cells isolated from colonic tissue (34). Trough plasma levels of TFV and FTC were lower by 32% (p = 0.010) and 32% (p = 0.038), respectively, in transgender women as compared to CGM. Similarly, the area under the curve (AUC) over 24 hours of TFV and FTC was lower by 27% (p = 0.065) and 24% (p = 0.028), respectively, in transgender women as compared to CGM. Other small studies have had varying results (35).
The potential implication of both the Baltimore and Thai studies is that dosing strategies less than daily (like on demand or event-driven strategies, in which PrEP is taken in the 48 hours surrounding a high-risk sexual encounter but not continuously) might confer less protection for transgender women taking GAHT, though this has not yet been directly studied. Transgender women might require tailored therapies and modified dosing regimens to ensure protection from HIV, but based on available data, it does not appear that PrEP impacts GAHT levels. These data suggest that daily dosing of TDF-based PrEP, in accordance with the label instructions, should offer transgender women excellent protection against HIV, in a manner that does not alter their hormonal levels or therapies.
Emtricitabine 200 mg/Tenofovir alafenamide fumarate 25 mg (F/TAF)
Tenofovir alafenamide (TAF) is another prodrug of tenofovir, which achieves lower plasma levels and higher intracellular metabolite concentrations than TDF, and has been less associated with renal and bone toxicities. The DISCOVER trial, of F/TAF as compared to F/TDF for HIV PrEP among individuals at high risk for HIV acquisition, demonstrated an overall HIV incidence rate of 0.16 infections/100 person-years in the TAF arm and 0.34 infections/100 person-years in the F/TDF arm (36). As compared to a background incidence of 4.2 HIV infections per 100 person-years, this represents a potential 96% relative risk reduction in the F/TAF arm.
Though no sensitivity analyses were presented in transgender women, nor were GAHT regimens described, the study still demonstrated a strong protective efficacy in this high-risk population. Neither TAF nor FTC have been shown to affect GAHT hormone concentrations, and indeed there is no known biologically plausible mechanism for this. The impact of testosterone ablation (orchiectomy and chemical ablation) on pharmacokinetics or efficacy of PrEP and ART is not well understood. Neither is the potential for transmission of HIV and other sexually transmitted infections during sexual exposures (or the impact of drug concentrations in such tissues on transmission) in the setting of a reconstructed neovagina.
Long-Acting Antiretrovirals
As transgender individuals might have a higher risk of inadequate access to employment and housing and thus poor access to health insurance and healthcare services, technologies that allow them to achieve highly efficacious treatment and prevention of HIV without the need for fastidious adherence to daily oral medications might stand to benefit them disproportionately. Long-acting nanoformulated injectable cabotegravir and rilpivirine have shown high efficacy for the treatment of HIV, and recent results from the HIV Prevention Trials Network (HPTN) 083 study showed that long-acting injectable cabotegravir was efficacious in preventing viral acquisition in both MSM and transgender women (37). Subgroup analyses of transgender women who participated in HPTN are forthcoming.
Transgender Men and PrEP
There is a lamentable paucity of data on the interactions of PrEP/ART and testosterone GAHT in transgender men, and transgender men have been excluded from many of the seminal trials on PrEP and antiretrovirals, including newer, long-acting antiretrovirals (38).
Other ART
Table 3 (available online at aacc.org/publications/cln) displays the risk of drug-drug-interactions (DDI) with GAHT. In general, much of what is known about interactions between sex hormones and ART comes from contraceptive interaction studies in women, which generally focus on ethinyl estradiol, etonogestrel, levonorgestrel, and norethisterone enanthate, different forms of estrogen from that used in GAHT (39–43). Some of the lessons are generalizable, though notably, many of the estrogen forms used in GAHT have not been directly studied with respect to common DDIs.
In general, non-nucleoside reverse transcriptase inhibitors like efavirenz reduce hormone concentrations via CYP 3A4 induction, with the exception of etravirine, which slightly increases the AUC of ethinyl estradiol (44, 45). Rilpivirine slightly increases the AUC and peak concentrations of ethinyl estradiol, which is not clinically relevant (39). Conversely, ritonavir- or cobicistat-boosted protease inhibitors increase the concentration of hormones via CYP 3A4 enzyme inhibition, with the single exception of estradiol, which is reduced by ritonavir, but not by cobicistat (39).
Conclusion
The interaction on the molecular level between exogenous hormones and antiretrovirals is still being studied. However, a sophisticated approach to the unique medical needs of trans people, including the appropriate laboratory monitoring of GAHT and sensitivity to the interplay between gender-affirming therapies and common pharmacotherapies, can and should inform the clinical care of transgender people and lead to optimization of drug management and clinical outcomes in this community, which has long been marginalized, overlooked, and underserved by the mainstream medical community.
Ethel D. Weld, MD, PhD, is an assistant professor of medicine, pharmacology, and molecular sciences at the Johns Hopkins University School of Medicine in Baltimore. She specializes in infectious diseases and clinical pharmacology. +Email: [email protected]
References
- Coleman E, Bockting W, Botzer M, et al. Standards of care for the health of transsexual, transgender, and gender nonconforming people Version 7. Int J Transgend 2012;13:165-232
- Winter S, Diamond M, Green J, et al. Transgender people: Health at the margins of society. Lancet 2016;388:390-400.
- Toomey RB, Syvertsen AK, Shramko M. Transgender adolescent suicide behavior. Pediatrics 2018;142:e20174218.
- Quinn VP, Nash R, Hunkeler E, et al. Cohort profile: Study of Transition, Outcomes and Gender (STRONG) to assess health status of transgender people. BMJ Open 2017;7:e018121.
- Winter S, Settle E, Wylie K, et al. Synergies in health and human rights: A call to action to improve transgender health. Lancet 2016;388:318-21.
- Baral SD, Poteat T, Stromdahl S, et al. Worldwide burden of HIV in transgender women: A systematic review and meta-analysis. Lancet Infect Dis 2013;13:214-22.
- Metastasio A, Negri A, Martinotti G, et al. Transitioning bodies. The case of self-prescribing sexual hormones in gender affirmation in individuals attending psychiatric services. Brain Sci 2018;8:88.
- Mepham N, Bouman WP, Arcelus J, et al. People with gender dysphoria who self-prescribe cross-sex hormones: Prevalence, sources, and side effects knowledge. J Sex Med 2014;11:2995-3001.
- Lescure D, Paget J, Schellevis F, et al. Determinants of self-medication with antibiotics in European and Anglo-Saxon Countries: A systematic review of the literature. Front Public Health 2018;6:370.
- Giguere R, Frasca T, Dolezal C, et al. Acceptability of three novel HIV prevention methods among young male and transgender female sex workers in Puerto Rico. AIDS Behav 2016;20:2192-202.
- Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: An Endocrine Society clinical practice guideline. Endocr Pract 2017;23:1437.
- Coleman E, Bockting W, Botzer M, et al. Standards of care for the health of transsexual, transgender, and gender nonconforming people Version 7. Int J Transgend 2012;13:165-232
- Waxman DJ, Holloway MG. Sex differences in the expression of hepatic drug metabolizing enzymes. Mol Pharmacol 2009;76:215-28.
- Wolbold R, Klein K, Burk O, et al. Sex is a major determinant of CYP3A4 expression in human liver. Hepatology 2003;38:978-88.
- Gandhi M, Aweeka F, Greenblatt RM, et al. Sex differences in pharmacokinetics and pharmacodynamics. Annu Rev Pharmacol Toxicol 2004;44:499-523.
- Ketha H, Girtman A, Singh RJ. Estradiol assays–The path ahead. Steroids 2015;99:39-44.
- Labs MC. Estradiol, rapid, immunoassay, serum. Test Catalog 2019.
- Guo T, Gu J, Soldin OP, et al. Rapid measurement of estrogens and their metabolites in human serum by liquid chromatography-tandem mass spectrometry without derivatization. Clin Biochem 2008;41:736-41.
- Zhou Y, Wang S. A robust LC-MS/MS assay with online cleanup for measurement of serum testosterone. J Sep Sci 2019;42:2561-8.
- Toishi Y, Tsunoda N, Nagata SI, et al. Evaluation of the chemiluminescent enzyme immunoassay system for the measurement of testosterone in the serum and whole blood of stallions. J Reprod Dev 2018;64:41-7.
- Zhou H, Wang Y, Gatcombe M, et al. Simultaneous measurement of total estradiol and testosterone in human serum by isotope dilution liquid chromatography tandem mass spectrometry. Anal Bioanal Chem 2017;409:5943-54.
- Xu W, Li H, Guan Q, et al. A rapid and simple liquid chromatography-tandem mass spectrometry method for the measurement of testosterone, androstenedione, and dehydroepiandrosterone in human serum. J Clin Lab Anal 2017;31:e22102.
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999;84:3666-72.
- Anderson PL, Reirden D, Castillo-Mancilla J. Pharmacologic considerations for preexposure prophylaxis in transgender women. J Acquir Immune Defic Syndr 2016;72 Suppl 3:S230-4.
- Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012;367:423-34.
- Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012;367:399-410.
- Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010;363:2587-99.
- Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2013;381:2083-90.
- Volk JE, Marcus JL, Phengrasamy T, et al. No new HIV infections with increasing use of HIV preexposure prophylaxis in a clinical practice setting. Clin Infect Dis 2015;61:1601-3.
- McCormack S, Dunn DT, Desai M, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): Effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet 2016;387:53-60.
- Anderson PL, Glidden DV, Liu A, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med 2012;4:151ra25.
- Deutsch MB, Glidden DV, Sevelius J, et al. HIV pre-exposure prophylaxis in transgender women: A subgroup analysis of the iPrEx trial. Lancet HIV 2015;2:e512-9.
- Hiransuthikul A, Janamnuaysook R, Himmad K, et al. Drug-drug interactions between feminizing hormone therapy and pre-exposure prophylaxis among transgender women: The iFACT study. J Int AIDS Soc 2019;22:e25338.
- Shieh E, Marzinke MA, Fuchs EJ, et al. Transgender women on oral HIV pre-exposure prophylaxis have significantly lower tenofovir and emtricitabine concentrations when also taking oestrogen when compared to cisgender men. J Int AIDS Soc 2019;22:e25405.
- Cottrell ML, Prince HMA, Schauer AP, et al. Decreased tenofovir diphosphate concentrations in a transgender female cohort: Implications for HIV pre-exposure prophylaxis (PrEP). Clin Infect Dis 2019;69:2201-4.
- Hare B, Coll P, Ruane P, et al. The Phase 3 DISCOVER Study: Daily F/TAF or F/TDF for HIV preexposure prophylaxis (Abstract 104). Conference on Retroviruses and Opportunistic Infections (CROI), Seattle, WA. 2019.
- Landovitz R. HPTN 083 Final Results: Pre-exposure prophylaxis containing long-acting injectable cabotegravir is safe and highly effective for cisgender men and transgender women who have sex with men. AIDS 2020.
- Poteat TC, Radix A. HIV antiretroviral treatment and pre-exposure prophylaxis in transgender individuals. Drugs 2020;80:965-72.
- Radix A, Sevelius J, Deutsch MB. Transgender women, hormonal therapy and HIV treatment: A comprehensive review of the literature and recommendations for best practices. J Int AIDS Soc 2016;19:20810.
- Nanda K, Stuart GS, Robinson J, et al. Drug interactions between hormonal contraceptives and antiretrovirals. AIDS 2017;31:917-52.
- Scarsi K, Lamorde M, Darin K, et al. Efavirenz but not nevirapine-based antiretroviral therapy decreases exposure to the levonorgestrel released from a sub-dermal contraceptive implant. J Int AIDS Soc 2014;17:19484.
- Scarsi KK, Darin KM, Nakalema S, et al. Unintended pregnancies observed with combined use of the Levonorgestrel contraceptive implant and Efavirenz-based antiretroviral therapy: A three-arm pharmacokinetic evaluation over 48 weeks. Clin Infect Dis 2016;62:675-82.
- Sevinsky H, Eley T, Persson A, et al. The effect of efavirenz on the pharmacokinetics of an oral contraceptive containing ethinyl estradiol and norgestimate in healthy HIV-negative women. Antivir Ther 2011;16:149-56.
- Leinung MC, Miller CH, Tehrani B, et al. The effect of Efavirenz on estradiol metabolism in transgender women. Transgend Health 2019;4:197-9.
- Scholler-Gyure M, Kakuda TN, Woodfall B, et al. Effect of steady-state etravirine on the pharmacokinetics and pharmacodynamics of ethinylestradiol and norethindrone. Contraception 2009;80:44-52.
- Das B, Dobrowolski C, Luttge B, et al. Estrogen receptor-1 is a key regulator of HIV-1 latency that imparts gender-specific restrictions on the latent reservoir. Proc Natl Acad Sci U S A 2018;115:E7795-E804.
- Centers for Disease Control and Prevention. Interim guidance: Preexposure prophylaxis for the prevention of HIV infection in men who have sex with men. MMWR Morb Mortal Wkly Rep 2011;60:65-8.
- Centers for Disease Control and Prevention. Update to interim guidance for preexposure prophylaxis (PrEP) for the prevention of HIV Infection: PrEP for injecting drug users. MMWR Morb Mortal Wkly Rep. 2013;62:463-5.
- Recommendations for HIV prevention with adults and adolescents with HIV in the United States, 2014.