Perhaps one of the most widely discussed issues in laboratory medicine is direct access testing (DAT), its impact on patient care, its cost, and the role of the laboratory professional. It is also referred to as co-ordered testing, patient-directed testing, direct access to lab services, consumer-driven testing, self-ordering, direct to consumer (DTC) testing, and consumer self-ordered testing. It is characterized by an individual requesting tests without a physician’s order and paying up front for this service, which is not usually reimbursed by insurance companies or other payers. Thirty-seven states and the District of Columbia allow direct access testing in some form, either with or without restrictions (1).
CLIA regulations do not distinguish between facilities performing direct access testing and those performing provider-ordered testing. CLIA certification must be maintained and CLIA laboratory procedures must be followed throughout all aspects of testing. In other words, CLIA only authorizes the regulation of the laboratories conducting testing and not the individuals who order or receive test results.
In the age of the internet and social media, consumers are seeking more control over their medical care and are willing to pay for this service. The reasons often cited for ordering tests include convenience, preparing for a doctor visit, saving money, privacy, getting a second opinion, and obtaining tests not covered by insurance.
The positive side of this trend is that consumers can gain useful information about laboratory tests from a variety of reputable sites, including AACC's Lab Tests Online, the National Academy of Clinical Biochemistry’s laboratory practice guidelines, public offerings from the College of American Pathologists, and many others including test catalogs from reference laboratories.
Retail outlets such as Walmart, Sam’s Club, Costco, and others are offering one-stop healthcare services including onsite testing for glucose and cholesterol, as well as tests that are subsequently sent out to reference laboratories. And telemedicine kiosks are available in some outlets with access available to physicians either by telephone or internet.
In this review, we plan to explore the impact of technology, public health implications, as well as perspectives from consumers, physicians, and the role of the laboratory professional in this environment.
The Impact of Technology on Direct To Consumer Testing
The recent move to electronic health records (EHR) has paved the way for sharing information through web portals. Wireless technology through mobile apps and wearable devices can track healthcare information in real time.
In both direct access testing and in the realm of patient portals, there is a new paradigm in healthcare where patients receive and often interpret their laboratory results in advance of their care providers. The major difference for patient portals, however, is that these laboratory tests are ordered by clinicians and should fall into the categories of screening, treatment monitoring, and/or disease-specific information.
Miniaturization of equipment, improved connectivity, and easier access to healthcare services are driving this field aggressively. Laboratory analytics that use microfluidic ELISA platforms, nanotechnology, biotechnology, and microelectronics are being applied in various devices to enable testing away from the central laboratory—or in the lab in an expedited amount of time. “Lab on a chip” as well as “Lab in a brief case” (2,3) that allow testing for a variety of analytes are being marketed worldwide.
These technical advances have resulted in a marked expansion of the list of CLIA waived tests. This means these tests can be used at the point of care either in a doctor’s office or in a patient’s home, including tests for microbial pathogens. For example, as of February 2018, there are 13 Food and Drug Administration (FDA)-cleared rapid influenza tests available in the U.S.: six are antigen-based and seven are nucleic acid-based.
Rapid nucleic acid amplification and detection techniques are being used to detect pathogens such as influenza, respiratory syncytial virus, and group A streptococci in just minutes (4-6). Based on an in-depth review of different rapid testing platforms for the detection of influenza, WHO (7) has concluded that the overall sensitivity is lower than cell culture (median 70-75%) while overall specificity is high (median 90-95%). Due to the low sensitivity of these tests, false negatives are a major concern requiring specimens being sent to the laboratory as well.
While rapid antigen tests for C. trachomatis and N. gonorrhoeae lack enough sensitivity to be used for screening, random-access, nucleic acid amplification-based platforms provide accurate point of care results in one hour, thus they have the potential to transform management and control of sexually transmitted diseases (STDs) in many endemic settings, including low/middle-income countries (8). Lateral flow immunoassays (LFIA) have been CLIA waived for the detection of HIV antibodies in fingerstick or venipuncture whole blood, as well as oral fluid (9).
A Simplified Process for the Consumer
Shoppers can search for tests based on specific symptoms or test categories including wellness, men’s and women’s health, and genomic health. Most popular test panels include food or allergen sensitivity, thyroid, metabolism, Vitamin D, and inflammation. There is a growing list of players in this field including Quest Diagnostics, LabCorp, 23andMe, Sonora Quest Labs, EverlyWell, and reference and private labs.
In addition, consumer genomic testing is gaining popularity with genomic labs partnering with large reference labs; however, these DTC tests come with warnings to educate the consumer that these tests are not be used for diagnosis or treatment decisions.
Shoppers first pay on the website for the test(s) requested. In some cases, this is followed by shipping a collection kit to the consumer which includes instructions for blood spot, saliva, or urine collection. In the case of HIV testing on oral fluid, patients are provided with clear instructions and an online video describing specimen collection. The kit is then returned by mail to the testing facility. Within a few days, a report will be provided, which may include a review by a board-certified physician.
In other cases, the patient goes to have their blood drawn at specific locations who then send that sample to a CLIA-certified lab like Quest or Labcorp. Labs like Quest may partner with providers to advise patients on next steps if test results are abnormal. The test results will be interpreted by the internet test center’s staff physician and emailed back directly and confidentially to the consumer.
Self-collected saliva specimens play an important role in public health crises, as they minimize the risk of exposing healthcare workers to pathogens. For example, SARS-CoV can be detected in saliva (10). In a study from Hong Kong, saliva specimens in sterile bottles were collected by patients and 11/12 patients were found to be positive for SARS-CoV-2. (11).
DAT providers particularly target testing for the microbial pathogens causing STDs, including HIV, Neisseria gonorrhoeae, Chlamydia trachomatis, and human papillomaviruses (HPV). DAT is attractive for such testing as there are significant barriers to doctor-ordered STD testing, including the inconvenience associated with time, location, and transportation; confidentiality concerns; and fear of social stigmatization.
In recent years, the resources available to support STD testing have decreased, resulting in reduced clinic hours and even clinic closures. Moreover, people tend to be nervous or embarrassed about talking to doctors about their sexual behavior or worried their doctors might judge them if they request STD testing.
DTC Public Health Implications
Several organizations including the FDA, Centers for Disease Control and Prevention (CDC), and the United State Preventive Services Taskforce have suggested that direct access testing can be used effectively in a few screening scenarios (12). These tests include lipid panels, glucose, HIV testing, and Hemoglobin A1c. The CDC does emphasize that these tests are not to be used for diagnostic purposes and to be used in conjunction with a medical provider.
DAT has been increasingly used for the detection of STD pathogens. DATs developed utilizing non-invasive, easy-to-collect specimens have been successful in detection of HIV as well as other STD pathogens. HIV self-test kits have the potential to increase testing rates around the globe and thereby lead to reductions in HIV morbidity and mortality (13). Several studies assessed the feasibility and acceptability among women of self-collecting cervicovaginal samples at home, returning samples by mail for HPV testing, and receiving HPV results by telephone or email (14).
According to a recently published systemic review and meta-analysis, for chlamydia testing in comparison to clinician-collected cervical swabs, sensitivity and specificities were 92% and 98% for vaginal swab and 88% and 99% for urine, respectively. For gonorrhea testing, self-collected urine samples compared to clinician-collected urethra samples in males produced a sensitivity of 92% and specificity of 99% (14). Studies in both high- and low-resource settings have found HPV self-testing to be well accepted and accurate as an initial screening test, with follow-up of positive self-test results (14).
No review of DAT is complete without at least a bird’s eye view of genetic testing. Much has been written and discussed about this at various forums. The FDA in 2017 allowed marketing of 23andMe Personal Genome Service Genetic H10 diseases/conditions (15). 23andMe genetic health risk (GHR) tests work by isolating DNA from a saliva sample that is tested for more than 500,000 genetic variants including three specific variations in BRCA1 and BRCA 2 genes and a test for Alzheimer’s disease by analyzing the APOE gene.
These tests provide information on an individual’s genetic predisposition for certain medical diseases or conditions which may help to make lifestyle choices or to be discussed with a healthcare professional. The FDA clearly indicated that the results of Genetic Health Risk (GHR) tests should not be used for diagnosis or to inform treatment decisions.
At present several companies offer DTC tests. These tests include estimating disease risk and health, ancestry or genealogy, kinship, and lifestyle. The benefits of these tests, apart from the financial aspects and not requiring physician involvement, include awareness of genetic diseases and the ability to be proactive about one’s health. On the other hand, the risks and limitations need to be considered (16). Most important is the fact that there is little oversight or regulation of testing and there is not enough scientific evidence linking many genetic variations with specific diseases or traits.
Although DTC specimen collection, test ordering, and reporting have been extremely simplified, one needs to interpret these results with caution and generally with assistance of a physician and/or genetic counselor.
The Physician’s Lens
To use HIV as an example, the debate on whether to take the doctor or healthcare provider out of test-ordering continues. The argument for including the physician often is to have a doctor’s involvement when the patient gets the report. In contrast, others do not think it is necessary to involve the physician in test ordering for patients savvy enough to order a test (17).
Despite the advantages of an HIV DAT that can be performed by nonprofessionals and at home using self-collected specimens in a proper manner, there remain significant limitations due to the lack of sensitivity, operator proficiency, and variability in oral fluid collection. Moreover, it has been shown that testing performed in a clinical laboratory is better than either waived oral fluid or fingerstick collection to detect HIV-1 infection.
In addition to technical weaknesses, other social and psychological concerns with DTC testing have been raised: unscrupulous laboratories might take advantage of consumers’ health worries through fear tactics, consumers may feel a false sense of security or panic for results that are falsely negative or positive, or possible legal restrictions to accessing home-testing. Often this results in additional specimens being sent to the traditional clinical laboratory.
In a hypothetical example, assume alpha fetal protein (AFP) is an analyte offered in direct access testing. What might happen if a consumer has an AFP result slightly above normal? If the person searches the internet for information, he or she might become convinced of a diagnosis of hepatocellular carcinoma, which has a dismal prognosis, and thus experience significant anxiety. However, this consumer is likely to be completely normal.
An important concept to understand in the DTC setting is the concept of low pretest probability of disease. False positive results in laboratory tests with excellent sensitivity and specificity are more likely than true positives in the low pretest probability setting. There is a misconception that a test within the reference range means the patient is healthy and outside the reference range means the patient is ill (18). Current guidelines do not even recommend AFP testing in patients with chronic Hepatitis C Virus (HCV), where the pre-test probability is higher, let alone in the general population.
FIGURE 1 Comparison of the Traditional Healthcare Model Versus Direct Access Testing
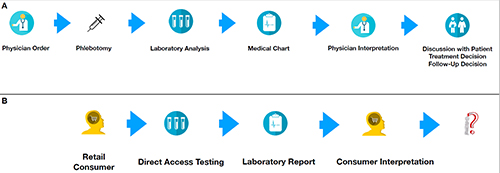
A: Represents the traditional paradigm in healthcare. B: New paradigm in DAT. Note that this process may create a great deal of confusion and anxiety for the individual. In many cases, the next step may be to make a doctor’s appointment and thus start back at “A”.
The Apple Watch EKG feature, recently cleared by the FDA, is another example of how technology can be applied directly to the consumer without regard for clinical utility and which will allow for constant testing of the general population. The FDA approval classifies the Apple Watch EKG app as an over-the counter, “informational use only” device like a single lead EKG. It is not intended to provide a diagnosis (19).
If this technology works accurately, and if the end goal is to inform the wearer when they have an abnormal heart rhythm, this concept again represents over-screening. Given the fact that many young people purchase Apple products, and since the prevalence of undiagnosed heart disease and electrophysiological pathology is very low in young people, this creates a major problem.
What happens when an abnormal heart rhythm is detected as a normal physiological response or the patient is an outlier, but otherwise healthy? These individuals would not normally be brought to clinical attention because they don’t have the clinical symptoms of shortness of breath, palpitations, dizziness, etc. In other words, most of these individuals are completely healthy.
Such over-testing can potentially create a huge burden on the healthcare system and cause unnecessary anxiety for these individuals. By the same token, what if an individual with no symptoms of hypercalcemia and is otherwise healthy decides to order a comprehensive metabolic panel (CMP) because it is being advertised in a pharmacy or another retail location? What if the person falls into the 2.5% of individuals outside of the reference range? Again, he or she will have unnecessary worry/anxiety and burden the healthcare system with low-value medical care.
In addition, it is likely that their treating physician will want to re-order some tests to confirm the results: The person’s physician may not feel comfortable trusting a consumer product, and good clinical practice would require confirmation of any abnormal outside laboratory findings prior to initiating treatment, particularly ones without confirming clinical findings or symptoms (See Figure 1).
A Challenge to the Laboratory and the Role of the Laboratorian
In the ongoing debate of the implications of direct access testing, the question arises as to the role of the laboratorian. For us, it is crystal clear where our expertise is, and we are probably the best equipped to provide guidance on the interpretation of these tests. However, for the consumer, we are an invisible and unknown entity. As a group our voices need to be heard and accessible over the web or the phone.
As clinical labs, we also need to examine our internal and external processes to try to be more “consumer friendly”. Our lab reports and their presentation can certainly be improved to make them more understandable to the consumer. Visual graphics are often easier to comprehend than tables of numbers and complicated text. Our websites and patient portals need to be more user-friendly and intuitive, with access when requested to a laboratory professional.
Although many institutions have consumer-friendly client service professionals, we as laboratorians can attempt to be more accessible to the patient as we continue our commitment to quality. It is also well known that traditional testing often requires the collection of large amounts of blood from our patients, and as an industry we need to try to come up with methods to offer more tests with less serum or plasma. We can encourage adoption of the new technologies outside of the traditional laboratory setting that are pushing towards using smaller quantities of blood.
The question often is raised as to how DAT compares with standard clinician-ordered laboratory tests performed in a CLIA-accredited laboratory. One argument to be made in favor of DAT is that this testing can be performed in CLIA accredited laboratories with the same stringent regulations as those ordered by clinician-ordered tests. While this may occur, the differences in methodologies and reference ranges still need to be reconciled when results from tests performed in different places are compared.
While there is a subset of people for whom DAT will improve their healthcare, access to STD testing being a major one (10), we believe that in many cases, DAT will create significant user anxiety and increased costs for the healthcare system, with very little benefit to users. When laboratory tests are ordered, and results interpreted without physician input, the results may not be an overall improvement to users’ health.
However, the drive for DAT appears to be increasing as people want more control over their own health. This is particularly a concern among the so-called worried well. These are individuals who need to constantly monitor their health despite no evidence of actual disease. The question remains to be answered: Does DAT result in a net gain or net loss in helping people to be healthier?
Acknowledgement: This study was funded in part through the National Institute of Health and National Cancer Institute Cancer Center Support Grant P30CA008748.
Lakshmi V. Ramanathan, PhD, is chief of clinical chemistry service at Memorial Sloan Kettering Cancer Center in New York, NY. Email: [email protected]
References
- AACC Report: Direct-to Consumer test results should be more patient-friendly. Lab Medica International 2015; 32 (6): 1.
- Barbosa AI, Castanheira AP, Edwards AD, et al. A lab-in-a-briefcase for rapid prostate specific antigen (PSA) screening from whole blood. Lab Chip 2014; 14:2918
- Whitesides G. The lab finally comes to the chip. Lab Chip 2014; 14: 3125.
- Wang H, Deng J, Tang YW. Profile of the Alere i Influenza A & B assay: a pioneering molecular point-of-care test. Expert Rev Mol Diagn. 2018; 18:403-409
- Brendish NJ, Schiff HF, Clark TW. Point-of-care testing for respiratory viruses in adults: The current landscape and future potential. J Infect. 2015; 71(5):501-10. doi: 10.1016/j.jinf.2015.07.008. Review.
- Ling L, Kaplan SE, Lopez JC, Stiles J, Lu X, Tang YW. Parallel Validation of Three Molecular Devices for Simultaneous Detection and Identification of Influenza A and B and Respiratory Syncytial Viruses. J Clin Microbiol. 2018 Feb 22;56(3). pii: e01691-17. doi: 10.1128/JCM.01691-17. Print 2018 Mar
- WHO recommendations on the use of rapid testing for influenza diagnosis, 2005.https://www.who.int/influenza/resources/documents/RapidTestInfluenza_WebVersion.pdf
- Causer LM, Guy RJ, Tabrizi SN, Whiley DM, Speers DJ, Ward J, et al. Molecular test for chlamydia and gonorrhea used at point of care in remote primary healthcare settings: a diagnostic test evaluation. Sex Transm Infect. 2018; 94:340-345.
- Pant Pai N, Sharma J, Shivkumar S, Pillay S, Vadnais C, Joseph L, et al. Supervised and unsupervised self-testing for HIV in high- and low-risk populations: a systematic review. PLoS Med 2013;10: e1001414
- Wang WK, Chen SY, Liu IJ, et al; SARS research group of the National Taiwan University/National Taiwan University Hospital. Detection of SARS-associated coronavirus in throat wash and saliva in early diagnosis. Emerg Infect Dis 2004;10: 1213-9.
- To KK, Tsang OTY, Yip CCY; Consistent detection of 2019 Novel Coronavirus in Saliva: Clinical Infectious Diseases, ciaa149, https://doi.org/10.1093/cid/ciaa149
- https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm624726.htm
- Stevens DR, Vrana CJ, Dlin RE, Korte JE. A Global Review of HIV Self-testing: Themes and Implications. AIDS Behav. 2018; 22:497-512.
- Lunny C, Taylor D, Hoang L, Wong T, Gilbert M, Lester R, Krajden M, Ogilvie G. Self-Collected versus Clinician-Collected Sampling for Chlamydia and Gonorrhea Screening: A Systemic Review and Meta-Analysis. PLoS One. 2015 Jul 10(7): e0132776
- https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm551185.htm
- Genetics Home Reference: Help me understand genetics, direct to consumer genetic testing. U.S. National Library of Medicine, National Institutes of health, Oct 2, 2018
- https://www.kansas.com/news/business/health-care/article1126188.html
- Astion ML. Quackery interventions: the hopelessness and the hope. Laboratory Errors and Patient Safety. 2007; (4): 2-5.
- https: //www.accessdata.fda.gov/cdrh docs/pdf18/eden180044.pdf