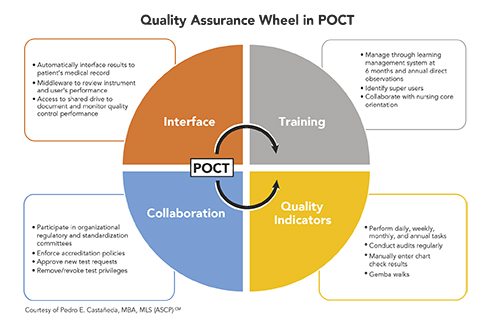
Effective quality assurance (QA) in point-of-care testing (POCT) requires a comprehensive, interdepartmental approach. Key elements for success include focusing clearly on quality indicators (QI), harnessing technology, training and recertifying current operators, and fostering proactive collaboration among users supported by trained POCT personnel.
POCT tracks with widespread and evolving patient care protocols. We have experienced this growth at University of Minnesota Medical Center, M Health Fairview, an integrated academic and research institution in Minneapolis that comprises three hospitals with a 1,057 bed capacity, free-standing and hospital-based clinics, inpatient and outpatient specialty hospital units, emergency departments, a surgery center, and research operations. Throughout these care sites, we offer 30 types of POCT, supporting approximately 4,000 healthcare providers.
My POCT colleagues and I have implemented an approach to ensuring POCT QA regulatory compliance that functions effectively in our large and diverse organization.
Managing What We Measure
The foundation of our plan rests on defining, monitoring, and assessing QI. We’ve created statistical measures across testing platforms to capture the performance of QI. Examples of measurable QI include labeling of reagent bottles with expiration dates, auditing the cleaning and disinfecting of POCT instruments, surveilling instrument errors in relationship to operator error and testing material wastage, and consistently documenting quality control (QC) of testing materials according to manufacturers’ instructions.
We also actively participate in our organization’s regulatory and standardization committees. Developing lines of communication through these committees has been essential in enabling us to provide technical advice. Our presence at these meetings also has helped us obtain senior nursing and medical leadership support in approving and enforcing POCT regulatory compliance.
Additionally, we thoroughly understand The Joint Commission’s and College of American Pathologists’ (CAP) governing guidelines and educate end users about appropriately using these regulations.
Scheduling Tasks, Reviewing Procedures
We create, monitor, and document completion of daily, weekly, monthly, biannual, and annual tasks related to regulatory compliance. We developed these tasks based on POCT manufacturers’ requirements for testing and in accordance with hospital accrediting agencies. For example, we perform QC on new lots and new shipments of testing materials, calibrate POCT instruments monthly, and certify operators annually.
Another aspect of our QA involves performing Gemba walks through hospital units and clinics. This element of Lean and Six Sigma consists of observing how POCT processes take place, requesting feedback from providers, and delivering answers and solutions to concerns or problems that might arise.
We maintain QA compliance by reviewing POCT procedures biennially or as needed to address important changes, ensuring that we incorporate into our procedures the technical verbiage in CAP checklists.
We’ve taken several steps to ensure that POCT operators have comprehensive, upfront training and maintain their competency. We provide regulatory and technical support to M Health Fairview’s nursing core orientation, and we periodically audit and assess POCT-related training and procedures. We also identify proficient and reliable employees to function as preceptors who observe, evaluate, and certify users in correctly performing the annual testing process.
We couple upfront compliance expectations with a three-step process for warning POCT users that their competencies are overdue and privileges will be suspended if their competencies are not completed. The process starts with an email reminder to each user and his or her manager. If necessary, we send a second email to the user, his or her manager, and director. After the second warning, the user will be locked out from performing POCT. In some cases, a test might be stopped or removed from a unit until a process improvement plan is established to restore compliance.
Using Technology
Tracking compliance manually for approximately 4,000 users would simply be impossible. We use a learning module system (LMS) to assign certification lessons for waived and non-waived testing, respectively, and review LMS reports monthly to assess compliance. In the case of waived testing, we follow up with QC performance at least once each year. For non-waived testing, we schedule annual observations during skills fair day, through individual arrangements, or during monthly faculty meetings.
We also take full advantage of POCT instrumentation interfaces and software configurations to facilitate compliance monitoring. For example, we utilize a POCT middleware alert system designed with at least two elements of compliance to remind staff of their certification expiration dates and the need to review their annual learning module. We also deploy instrument lock-out settings to ensure end user compliance, use instrument interfaces to transmit results to medical records, and monitor QC through the Levey and Jennings function.
Another way we harness technology is by creating and implementing interactive, remotely accessed electronic QC logs. These handy metrics feature pop-up alerts and messages to end users to ensure QC and regulatory compliance.
A feasible, clear, and comprehensive action plan—effectively shared and implemented across the organization—is vital to sustaining POCT compliance. In a successful strategy, all processes performed are interconnected and interdependent in a synergistic relationship that ensures the efficacy of the approach.
Pedro E. Castañeda, MBA, MLS (ASCP)CM, is point-of-care testing supervisor at University of Minnesota Medical Center, M Health Fairview in Minneapolis. +Email: [email protected]
The author gratefully acknowledges the participation of Ryan J. Strand, MLS (ASCP)CM, Sheree Humphries, MLS (ASCP)CM, Carmen L. Dorschner, MLT (ASCP), and Jessica Kopecky, MLS (ASCP)CM , in their daily POCT work and in writing and reviewing this article, with special thanks to Chris Senn, MLS (ASCP)CM.