Chronic kidney disease (CKD) is the ninth leading cause of death in the U.S. with an estimated 14.2% of the U.S. population presenting evidence of CKD stages 1-4 (1). This disease affects African Americans at a rate three times higher than Caucasians and has been termed the “silent killer” because it shows no symptoms in early stages, and many physicians and patients remain unaware of the diversity of its clinical characteristics (2).
To address these issues globally, the international organization Kidney Disease: Improving Global Outcomes (KDIGO) issued evidence-based clinical practice guidelines in 2013 with clear criteria for diagnosing CKD (3). KDIGO defines CKD as abnormalities of kidney structure or function present for more than 3 months. Abnormalities include one of two factors: either a marker of kidney damage, such as urine sediment abnormalities or albuminuria, or decreased glomerular filtration rate (GFR) (< 60 ml/min/1.73 m2).
Glomerular Filtration Rate: Essential and Elusive
GFR, which represents the total amount of blood filtered through the glomerulus in the kidney, is the best overall index of kidney function. KDIGO has established six categories for GFR (Table 1) based on the severity of kidney dysfunction (3).
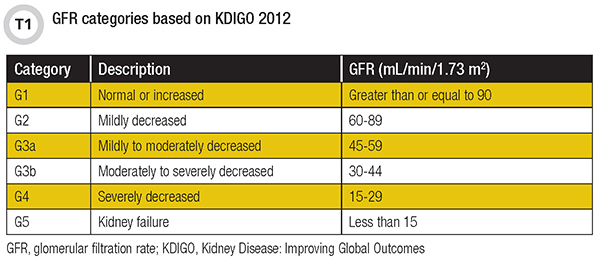
GFR can only be determined by measuring the clearance of a substance from blood into urine (4). However, for the clearance of this substance to truly reflect GFR, the substance must meet the following characteristics:
- Freely filtered at the glomerulus;
- Produced at a constant rate, so stable blood concentrations are maintained;
- Neither secreted nor reabsorbed by the renal tubules;
- No extra-renal elimination pathways; and
- Physiologically inert.
To date, no single known biomarker produced by the human body meets all these characteristics. As a result, laboratories use clearance of exogenous markers such as iothalamate and iohexol as a reference method for determining GFR, while using serum measurement of endogenous markers such as creatinine and cystatin C for estimating GFR (eGFR).
eGFR Equations and Their Limitations
The expense and invasiveness of assessing kidney function using exogenous markers makes doing so impractical for screening purposes. Consequently, clinical laboratories rely on serum creatinine to screen for kidney disease. Creatinine is the waste product of interconversion between phosphocreatine and creatine in muscle cells. Thus, the amount of creatinine produced in an individual every day is fairly constant and correlates with muscle mass. However, muscle mass varies widely among individuals, and creatinine varies significantly by age, sex, and race.
Several equations have been developed to account for these variations, such as Modification of Diet in Renal Disease (MDRD) and more recently the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI). But while these equations improve accuracy, they do not account for other non-renal factors that affect creatinine, such as diet, certain medications, and amputations (4). In patients with these factors, as well as in those with abnormally low or high muscle mass, cystatin C is the recommended marker for eGFR.
Cystatin C has been shown to be superior to creatinine as a marker of kidney function and for eGFR (5). However, cystatin C is also influenced by non-renal factors such as inflammation, thyroid function, and obesity (4), and is a much more expensive test than creatinine. For these reasons it has limited use in clinical practice, though recent guidelines recommend it to confirm CKD among individuals with eGFR 45-59 ml/min/1.73 m2.
SDMA Biomarker Characteristics
Symmetric dimethylarginine (SDMA) is an emerging endogenous biomarker of kidney function that is already widely used in veterinary medicine. Asymmetric dimethylarginine (ADMA) and SDMA both are produced consistently by hydrolysis of histones with post-translational methylation of arginine residues. In addition, SDMA is almost exclusively eliminated by the kidneys after filtration, making it an ideal candidate for a GFR marker.
ADMA and SDMA have been known to biochemists for decades. ADMA emerged as a marker of endothelial dysfunction, while SDMA had been left in the shadows as a structural isomer of ADMA with no clinical utility. It took many years for SDMA to attract attention, until the early 2000s when in back-to-back studies it surprised researchers as an independent cardiovascular disease (CVD) risk factor along with ADMA. Furthermore, a meta-analysis of 18 studies published in 2006 showed a highly significant correlation between SDMA and kidney function (6). These studies also reported no effect from non-renal factors known to affect creatinine and/or cystatin C, such as muscle mass, diet, inflammation, diabetes, and estrogen therapy. Importantly, in the absence of kidney disease, SDMA concentrations were not affected by hepatic disease, CVD, diabetes, or an acute inflammatory response.
SDMA has other advantages as a biomarker, including its low intra-individual biological variability (5.8%) in comparison with cystatin C (8.6%), and that unlike creatinine, no racial differences have been observed in studies examining SDMA levels in Caucasians versus African American men. On the other hand, SDMA levels have been shown to be slightly increased based on age and sex, so it is likely that a GFR estimating equation using SDMA would need to take into account at least these two variables.
SDMA and Kidney Function
A significant number of studies have reported strong correlations between SDMA and measures of kidney function in humans (7). However, only a handful compared the performance of SDMA to measured GFR using gold-standard or well-characterized exogenous biomarkers such as inulin, iothalamate, or chromium-EDTA (Table 2). These studies reported correlations ranging from 0.78 to 0.90 and included healthy as well as CKD and type 1 diabetes populations.
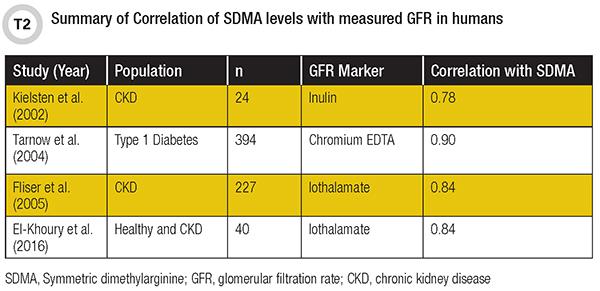
In 2016, our group published the first report comparing the performance of SDMA, creatinine, and cystatin C to measured GFR (8). Although the sample size was small (n=40), SDMA showed similar correlation with GFR as reported in larger studies (r=0.84). This was better than creatinine (r=0.70) but similar to cystatin C (r=0.86). Notably, we also included healthy and diseased populations in the comparison so that we were able to evaluate the performance of these markers across the spectrum of GFR values (13 to 151 mL/min/1.73 m2). Together, these studies demonstrate that SDMA is a strong marker of kidney function that highly correlates with GFR in adults.
Studies showing correlation of SDMA with measured GFR in pediatric populations are lacking, but several have examined correlations with eGFR. In one that included 35 patients with CKD and 42 healthy controls, SDMA had a higher diagnostic efficiency than cystatin C for detecting CKD and for identifying early stages of CKD (stages G1 and G2) (9).
Another important characteristic of SDMA is its early rise in the pathological process of kidney dis-ease. In a recent study examining changes in GFR markers after living-related kidney donation, SDMA increased within 6 hours after unilateral nephrectomy (i.e. 50% loss of kidney function) (10). While additional data in humans is lacking, SDMA has been shown to allow earlier detection of CKD in cats and dogs in comparison with creatinine.
Analytical Considerations
Measurement of SDMA has been largely restricted to translational research laboratories with liquid chromatography-tandem mass spectrometry (LC-MS/MS) instruments. While the adoption of LC-MS/MS by clinical laboratories has increased in recent years, the technology remains complex and requires a significant level of expertise for test development and operation. In addition, LC-MS/MS assays are not fully automated and require significant sample preparation and time before a result can be produced, increasing its turnaround time (TAT) in comparison with automated assays for cre-atinine and cystatin C. This lack of an automated assay has hampered SDMA’s widespread adoption in research and in clinical practice.
We recently evaluated the performance of a pre-commercial, research-use-only automated immunoassay for SDMA on two different platforms and compared its performance to LC-MS/MS. The new automated method showed strong correlation with LC-MS/MS on both platforms (unpublished data). The availability of an automated SDMA immunoassay with fast TAT raises the hope that this biomarker may be used in clinical practice in the future.
Future Perspectives
The commercial availability of an automated assay for SDMA is a high priority for the field as stake-holders seek to address with precise and reliable laboratory testing the growing burden of CKD and other chronic diseases. An automated assay will also speed adoption of SDMA measurement in translational and clinical laboratories and accelerate researchers’ ability to generate the data needed to fully characterize the biomarker for clinical use.
On the clinical side, it is important to investigate the non-renal factors that affect SDMA levels that may confound interpretation of results. Clinicians will also need an eGFR equation that establishes the relationship between SDMA and GFR and accounts for some of these confounding variables.
For now, it seems that the most promising application of SDMA is in the pediatric population, where it has already demonstrated superiority to cystatin C. However, further research along this line of investigation also is needed.
Finally, we should learn from the experiences of our colleagues in veterinary medicine, where this biomarker has been in routine clinical use since 2015. Their experience is especially relevant considering that normal SDMA levels are relatively consistent among humans, cats, and dogs. We have a lot in common with our furry friends, and we can use this knowledge to our advantage when it comes to measuring SDMA to assess kidney function.
*Dr. El-Khoury has received research support, as well as honorarium/expenses, from IDEXX Laboratories.
Joe M. El-Khoury, PhD, DABCC, FADLM is an assistant professor of laboratory medicine at Yale University School of Medicine and co-director of the clinical chemistry laboratory and clinical chem-istry fellowship program at Yale-New Haven Health in New Haven, Connecticut. +Email: joe.el-khoury[at]yale.edu
References
- Centers for Disease Control and Prevention. Chronic Kidney Disease Surveillance System—United States website. http://www.cdc.gov/ckd; accessed Sept. 10, 2018.
- Kopyt NP. Chronic kidney disease: the new silent killer. J Am Osteopath Assoc 2006;106: 133-6.
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO clinical prac-tice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013;3:1-150.
- Rifai N, Horvath AR, Wittwer CT, eds. Tietz Textbook of Clinical Chemistry and Molecular Di-agnostics, 6th ed. St. Louis, MO: Saunders Elsevier, 2018; chapter 32: page 506.
- Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum cre-atinine and cystatin C. N Engl J Med 2012;367:20-9.
- Kielstein JT, Salpeter SR, Bode-Boeger SM, et al. Symmetric dimethylarginine (SDMA) as en-dogenous marker of renal function—A meta-analysis. Nephro. Dial Transplant. 2006;21:2445–51.
- Schwedhelm E, Boger RH. The role of asymmetric and symmetric dimethylarginines in renal disease. Nat Rev Nephrol 2011;7:275–85.
- El-Khoury JM, Bunch DR, Hu B, et al. Comparison of symmetric dimethylarginine with creati-nine, cystatin C and their eGFR equations as markers of kidney function. Clin. Biochem 2016;49:1140-3.
- Wasilewska A, Taranta-Janusz K, Zoch-Zwierz W, Michaluk-Skutnik J. Is plasma symmetric dimethylarginine a suitable marker of renal function in children and adolescents? Scand J Urol Nephrol 2012;46:58-64.
- Kielstein JT, Veldink H, Martens-Lobenhoffer J et al. SDMA is an early marker of change in GFR after living-related kidney donation. Nephrol Dial Transplant 2011;26:324-8.