Often when clinical laboratorians think of toxicology testing, forensic, pre-employment, or pain management testing come to mind. Indeed these areas have conventionally been the focus for establishing cutoffs, policies and procedures for collection and analysis, method validation, and interpreting results. However, with the increase in opioid abuse over the last decade, drug abuse during pregnancy also has risen rapidly, boosting both the number of expectant mothers on opiate maintenance therapy and the incidence of neonatal abstinence syndrome (NAS) (1,2). As a result, assessing in utero drug exposure during pregnancy is emerging as a crucial service hospital laboratories provide for both patient care teams and social services. This is causing clinical laboratories to take a critical look at their policies and procedures.
Neonatal toxicology testing is a unique area of laboratory stewardship that encompasses managing test utilization, educating stakeholders, interpreting results, and contributing to the development of sound institutional policies. In contrast to most other forms of toxicology testing, a provider who orders a neonatal drug test is not the only individual acting on the result, which has cascading effects on treatments and social services. If a laboratory’s expertise remains at the nucleus of testing, we can contribute across all disciplines to create better continuity of care.
Universal Versus Risk-Based Testing
There are two major approaches to neonatal drug testing, and laboratories play a critical role in making sure that an organization’s testing strategy is evidence-based to reduce the potential for biased selection. Universal screening means consistently testing all newborns born at an institution. Risk-based screening means testing neonates only when a mother or a neonate meets hospital-defined criteria, which may include maternal history or signs of drug use, social risk factors, limited or absent prenatal care, and symptoms of withdrawal in the neonate.
There are advantages and disadvantages to both approaches. A universal testing program will generate more positive results that require follow-up by both laboratories and patient care teams. Likewise, the number of unexpected results may increase, requiring significant resources to investigate. More positive results also lead to increased referrals to social services and may place an undue burden on governmental agencies for relatively low-risk mothers.
Alternatively, risk-based testing may be perceived as unfairly profiling certain groups of patients, exposing an institution to potential legal issues. The risk-based testing approach may also miss drug-exposed neonates if the policy doesn’t adequately identify and test for probable exposures. These and other factors make risk-based testing policies more complicated to follow and monitor effectively. If risk-based testing has been implemented, proper assessment of risk factors must be completed by the obstetrics-gynecology team and communicated to the labor and delivery unit. The decision for or against testing must be made prior to delivery to ensure proper collection of the desired specimen.
Currently the American College of Obstetricians and Gynecologists (ACOG) recommends routine screening for substance abuse disorder for all individuals, but screening is defined as questionnaires or patient interviews. The ACOG guideline states that routine laboratory testing of biological specimens is not required (3). This leaves in the hands of individual institutions the decision to establish appropriate drug testing policies.
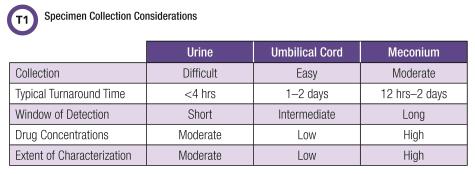
Specimen Selection and Collection
Neonatal toxicology testing presents distinctive pre-analytical challenges, most of which involve the difficulty in collecting adequate amounts of specimen to perform testing. For example, neonatal urine specimens may require waiting several hours after delivery. Likewise, the entirety of a baby’s meconium—the stool formed during gestation—must be collected even when passed in stages at several different times. Often laboratories receive an inadequate amount of meconium to perform necessary confirmatory testing, which may lead to testing being canceled due to quantity not sufficient. In the case of umbilical cord testing, specimens must be rinsed with saline to remove excess blood, then dried and stored prior to analysis. Laboratories need to review their specimen handling instructions for each specimen type used.
Laboratories also play a role in ensuring that providers in labor and delivery, newborn nurseries, and neonatal intensive care units (NICU) are aware of their responsibilities in specimen collection. The choice of specimen dictates when and where it will be collected: Umbilical cord tissue is collected during birth, while neonatal urine must be collected soon after birth if the first void is desired. Meconium requires collection whenever it is passed, which may occur shortly after birth or be delayed up to 48 hours or even later in some instances. Meconium may also be unavailable entirely if passed in utero. Ease of collection and other logistical considerations often influence an institution’s choice of specimen.
Urine
Although urine is the most common specimen in adult toxicology testing, it is used less frequently in neonates due to significant limitations. Collecting urine from a neonate can be challenging and often requires specialized collection devices. Removing urine from a diaper may contaminate the specimen. In fact, most neonates produce very little urine in their first few days of life. A recent study estimated urine output at 2.5 mL/kg/h or approximately 125 mL per day during the first 48 hours after birth. This explains why it can be nearly impossible to collect an adequate amount of specimen in a timely manner, even when using an appropriate collection device (4).
In addition to specimen collection problems, urine drug testing results can be of limited value in neonates. Urine typically provides a window of drug detection that spans only several days, and while this is extended slightly in neonates’ first void, it has low sensitivity for more distant exposures. Due to their immature livers, neonates may also produce unique drug metabolites. In addition, most urine drug screens rely on immunoassays that react across broad drug classes and are targeted primarily to metabolites produced in adult populations. Neonatal metabolites may not be the target compounds for immunoassay screens, and limited cross-reactivity can lead to false-negative results (5).
Meconium
Meconium is one of the most commonly used specimens for neonatal drug testing and is considered the gold standard specimen. Although meconium begins to form around the 12th week of gestation, more than half of the material is produced in the final 8 weeks of pregnancy. Many waste products, including drugs and metabolites, accumulate in meconium (6). This leads to meconium’s very long window of detection, which is estimated to include exposures in most of the third trimester, and in some cases, the later part of the second trimester (7).
Despite meconium’s long time use in neonatal testing, both laboratorians and other patient care team members face challenges in collecting it. Staff may encounter difficulties in obtaining adequate amounts of meconium and actually may not be able to if the meconium has been expelled in utero. Neonates may also pass meconium in stages, requiring multiple collections, and this passage may be delayed several days after birth. Because meconium is heterogeneous, all collections must be stored, combined, and mixed well prior to testing. However, prolonged storage can also impact the stability of drugs and metabolites. This makes proper meconium collection an onerous task for pa-tient care staff and sometimes leads to specimen mix-ups.
For these reasons, some institutions are exploring alternative types of specimens like umbilical cord tissue. Nevertheless, meconium is still considered the gold standard specimen for assessing long-term drug exposure in newborns.
Umbilical Cord Tissue
Umbilical cord tissue is a relative newcomer in the field of neonatal toxicology testing, but it has been adopted by a growing number of institutions. Unlike meconium, cord tissue is available immediately after birth and the collection process is simple. Consequently, toxicology testing of cord tissue often has a decreased time-to-results relative to meconium (8).
Despite these benefits, the mechanism of drug deposition into cord tissue is not well understood, and studies have found that drugs and metabolites do not accumulate in cord tissue to the same ex-tent as in meconium (9). Not surprisingly, the window of drug detection using a cord tissue sample is shorter than meconium, and cord tissue may not capture as many exposures as meconium (9-11).
Method Validation Requirements
There are no Food and Drug Administration-cleared or approved toxicology tests for meconium or umbilical cord tissue, so all tests in the marketplace are regulated as laboratory developed tests. For laboratories, this means increased demands during test development and validation. Both cord tissue and meconium require extensive pre-analytical sample preparation that often includes some form of homogenization or tissue disruption. Owing to the amount of labor required to process such specimens, these methods can be very expensive.
Several other issues further complicate method validation, including extensive sample cleanup and variable recovery of target analytes from complex mixtures like cord tissue and meconium. The limit of detection for a given drug also may vary substantially between laboratories.
The Importance of Turnaround Time
Turnaround time for neonatal drug testing has an outsize impact on how a variety of stakeholders use neonatal toxicology results, as it affects everything from discharge decisions to possible legal actions against a baby’s parents. Labs must deliver neonatal toxicology test results quickly, ideally within 48 hours of birth. Delaying discharge until results are available is not always possible, and this may pose problems for both hospitals and neonates. For example, if results that suggest ongoing maternal substance abuse arrive after a neonate has been discharged, this baby may experience additional preventable trauma before its mother has been re-contacted, or be lost entirely to fol-low-up by providers and social services.
Most hospital laboratories offer same-day results when they test urine. However, more complex specimens such as meconium or umbilical cord tissue are often sent to outside laboratories for analysis. The increased turnaround time for sendout tests may have real consequences for diagnostic management teams. Without timely neonatal toxicology results, providers assessing an infant for NAS symptoms may only have as a guide maternal history or maternal toxicology results.
Challenges in Result Interpretation
Providers often find interpreting neonatal toxicology results particularly challenging: They must integrate multiple patient results (maternal and newborn) and multiple specimen types (urine, meconi-um, and umbilical cord), while also evaluating medication lists and the mother’s self-reported history.
Moreover, providers must also integrate results from multiple testing laboratories that may have different methodologies or different limits of detection: A test result from one laboratory is not necessarily interchangeable with a result from another. This may be particularly problematic in states that require reporting of positive results to local, county, or state health departments, which in turn interpret the findings. Furthermore, a more sensitive analytical method may yield additional positive results, and if the effect of this on an institution’s positivity rate is not appreciated, a hospital’s population may appear to have a more substantial problem with maternal drug use than is truly the case.
Often these multiple result streams do not agree. In these cases, clinical laboratorians troubleshoot unexpected results using their expertise in toxicology testing to provide critical evaluation of the total testing process.
Providers also face challenges when they must act on unconfirmed or presumptive positive results. If a laboratory performs drug screening in-house and sends out the confirmation to a reference lab, providers must balance how and when to act on results. Presumptive positive results may trigger specific protocols to deal with NAS, but acting on an unconfirmed result may negatively affect the mother-baby relationship and patient-provider relationship.
Importantly, the renewed focus on the patient experience in hospitals also underscores the risk of false positives, which can very negatively influence public perception of the quality of care.
Stakeholders and Result Utilization
Neonatal drug testing programs require collaboration across a continuum of providers and stakeholders. The process of sample collection, the analytical performance of the test, and the composition of drugs detected all affect how stakeholders view the clinical performance. All providers—and clinical laboratorians—would prefer an affordable test with 100% sensitivity and specificity with a rapid turnaround time. However, real-world constraints dictate compromises related to sensitivity, cost, and turnaround time. Several publications have demonstrated that specimen type influences the specific drugs detected due to differences in distribution and deposition in the matrix (9).
Additionally, the analytical method and the limit of detection for target compounds prevent a specific specimen type or method from being ideal for all applications. Due to the current opioid epidemic, healthcare providers in NICUs and newborn nurseries may consider detection of opiates (illicit or prescribed) the most important component of the toxicology results profile. Adequate detection of opiates enables these stakeholders to rapidly triage infants who may develop NAS to the proper hospital unit and provide therapeutic intervention as soon as possible. Conversely, providers in social services may be concerned with non-opiate drugs such as cannabis, benzodiazepines, or gabapentin that have potential for abuse but are not typically associated with NAS.

Conclusion
Creating an effective neonatal testing program requires careful consideration of the preanalytic, analytic, and post-analytic phases of testing. The unique specimen types associated with neonates are distinct from those used for other toxicology testing applications such as pain management. Laboratories need to examine these differences carefully to ensure specimen quality and accurate timely results.
Neonatal testing is an opportunity for clinical laboratorians to assume a prominent role in the diagnostic management team for newborns, as laboratory testing has cascading impacts on treatment. Effective laboratory stewardship in this unique area highlights the role of laboratorians in providing effective healthcare.
Jennifer M. Colby, PhD, is associate director for clinical chemistry and an assistant professor in the department of pathology, microbiology, and immunology at Vanderbilt University Medical Center in Nashville, Tennessee. +Email: [email protected]
Steven W. Cotten, PhD, is director for chemistry, immunology, toxicology, and point-of-care testing at the Ohio State University Wexner Medical Center clinical laboratories and an assistant professor of pathology at Ohio State University in Columbus, Ohio. +Email: [email protected]
References
- Ohio Department of Health. 2015 Ohio drug overdose data report. http://www.healthy.ohio.gov/-/media/HealthyOhio/ASSETS/Files/injury-prevention/2015-Overdose-Data/2015-Ohio-Drug-Overdose-Data-Report-FINAL.pdf?la=en (Accessed December 13, 2016).
- Ohio Department of Health. NAS summary report 2012-2014. http://www.healthy.ohio.gov/-/media/HealthyOhio/ASSETS/Files/injury-prevention/NAS-Summary-Report-03-17b---Updated-03-22-2016-Final.pdf?la=en (Accessed December 13, 2016).
- Committee opinion no. 633: Alcohol abuse and other substance use disorders ethical issues in obstetric and gynecologic practice. Obstet Gynecol 2015;125:1529.
- Kasamatsu A, Ohashi A, Tsuji S, et al. Prediction of urine volume soon after birth using serum cystatin C. Clin Exp Nephrol 2016;764–9.
- Barakauskas VE, Davis R, Krasowski MD, et al. Unresolved discrepancies between canna-binoid test results for infant urine. Clin Chem 2012;58:1364–7.
- Gray TR, LaGasse LL, Smith LM, et al. Identification of prenatal amphetamines exposure by maternal interview and meconium toxicology in the Infant Development, Environment and Life-style (IDEAL) Study. Ther Drug Monit 2009;31:769–75.
- Himes SK, Tassiopoulos K, Yogev R, et al. Antiretroviral drugs in meconium: Detection for dif-ferent gestational periods of exposure. J Pediatr 2015;167:305–11.e3.
- Labardee RM, Swartzwelder JR, Gebhardt KE, et al. Method performance and clinical work-flow outcomes associated with meconium and umbilical cord toxicology testing. Clin Biochem 2017; doi:10.1016/j.clinbiochem.2017.09.016.
- Concheiro-Guisan A, Concheiro M. Bioanalysis during pregnancy: Recent advances and novel sampling strategies. Bioanalysis 2014;6:3133–53.
- Concheiro M, Lendoiro E, de Castro A, et al. Bioanalysis for cocaine, opiates, methadone, and amphetamines exposure detection during pregnancy. Drug Test Anal 2017;9:898–904.
- Colby JM. Comparison of umbilical cord tissue and meconium for the confirmation of in utero drug exposure. Clin Biochem 2017;50:784–90.