As with any analytical technique used routinely by clinical laboratory professionals, liquid chromatography-tandem mass spectrometry (LC-MS/MS)-based methods are subject to interference. Despite the superior analytical specificity of these methods, interference may arise at any point before or during the testing workflow—from patient treatment and preparation through phlebotomy
to analysis.
But with LC-MS/MS-based methods, labs have an advantage. Interference may be inevitable; however, labs have tools at their disposal during method development to optimize analysis and minimize the risk of result-altering interference. These tools include selective sample preparation, chromatography, and measuring multiple analyte-specific ions in the mass spectrometer. In addition, unlike clinical chemistry methods such as immunoassays, labs using LC-MS/MS benefit from monitoring several data quality metrics that help prevent potentially compromised test results from being released.
LC-MS/MS methods had been relegated to the world of reference measurement procedures, but more and more clinical chemistry labs are implementing them for routine testing. And although LC-MS/MS-based tests are neither infallible, nor by definition reference methods, labs have found that well-developed and well-controlled methods provide results superior to most other testing technologies in use today.
Understanding Interference
Analytical interference is the effect of a substance, identified or not, that causes the measured concentration of an analyte to differ from its true value (1). Method developers using LC-MS/MS need to consider many possible interferents. These include: compounds related to patient treatment (drugs, parenteral nutrition, plasma expanders); metabolites produced in pathological conditions; substances ingested by patients (alcohol, drugs of abuse, nutritional supplements, food); substances added during sample preparation (anticoagulants, preservatives, stabilizers); contamination during sample handling (hand cream, serum separators, collection tube stoppers, leachables from plastic consumables); and interferences arising from the sample matrix itself, such as hemolysis, icterus, and lipemia (2).
Whatever the source of interference, when defining acceptable interference levels labs need to consider the clinical context in which a test result will be used as well as the allowable analytical error limits. Interference may appear in an assay as partially or completely co-eluting peaks in the analyte or internal standard mass chromatograms. Interference may also be virtually invisible to technologists—a matrix effect.
Labs should be able to mitigate interference by taking advantage of three elements of selectivity in LC-MS/MS assays. The first, chromatographic separation, requires careful selection of column properties, mobile phase composition, and gradient elution profile. These provide the tools to manipulate the chromatographic retention of analytes in such a way that they elute in regions of reduced matrix effects and interference. Second, the relevant precursor ion is selected using the first quadrupole (MS1). Only ions having the same precursor ion mass enter the collision cell and are fragmented to produce smaller product ions. Finally, labs should be strategic in selecting analyte-specific product ion(s) using the second quadrupole (MS2).
This combination of precursor and product ion—a technique known as selected reaction monitoring (SRM) or multiple reaction monitoring (MRM)—and the ratio of two or more product ions yield a high degree of analytical selectivity. It is worth noting that any compounds with the same precursor and product ion(s) masses will be selected, leaving open the possibility for isobaric interferences.
Unlike these analytical interferences, matrix effects are caused by interference that alters the efficiency of the analyte and/or internal standard ions reaching the MS detector. Matrix effects often arise from the sample matrix and substances added to the matrix during sample handling and preparation.
Assessing Susceptibility to Interference
While labs are able to minimize interference, they rarely are successful in eliminating it completely (the ugly part). Consequently, clinical lab professionals absolutely need to assess susceptibility to analytical interference for any LC-MS/MS method development and validation: This interference affects method accuracy and precision, and therefore robustness. Interference testing falls into two categories: direct testing of the effect of specific substances on analyte concentration, and evaluation of unidentified interferences arising from sample matrix and anything added to it, i.e., matrix effects.
Testing for Specific Interference
As part of method development and validation, labs need to test method robustness in the presence of potential interferents in patient specimens, such as common sample abnormalities (hemolysis, icterus, lipemia), common prescription and over-the-counter drugs, medications prescribed for conditions for which the test is offered, dietary supplements, etc. The Clinical and Laboratory Standards Institute (CLSI) guideline, EP7-A2, section 5.4, offers recommendations for potential interfering substance selection (2). In LC-MS/MS, labs need to pay special attention to compounds that are isobaric with the analyte/internal standard. These compounds may interfere if not separated chromatographically.
Using this approach, described in detail in CLSI EP7-A2, a potential interferent is added to a sample pool and the target analyte concentration bias is evaluated relative to a control portion of the same sample pool (2). Labs then analyze both test and control pools in the same manner as patient specimens, with adequate replication, within one analytical run. Initially, the test substances are spiked at the highest concentration expected in patient specimens submitted for analysis. (Recommended test concentrations for many analytes and interferents are listed in EP7-A2.) When substances produce a clinically significant interference, labs should further evaluate these substances at different concentrations to determine the magnitude of the interference (3, 4).
This approach offers several advantages, including giving labs the ability to define acceptable/unacceptable sample collection conditions and abnormalities. Labs also should consider providing patient preparation guidelines to health practitioners who order the test in question. Guidelines that cover the medications, supplements, and foods to avoid prior to sample collection may help reduce repeated specimen collection and analysis. The disadvantages of this approach are the laboriousness of testing a large number of substances and the fact that no practical interference study will identify all potential interference.
Testing for Unidentified Interference
Unlike most other analytical techniques, LC-MS/MS enables labs to test for interference that cannot be anticipated or identified beforehand. Interference arising from sample matrix has the potential to cause either signal enhancement or, more commonly, signal suppression—whether the matrix effect is biological or added (salts, lipids, as well any of the substances mentioned previously). Labs can evaluate these matrix effects without identifying the specific substances causing them using either a quantitative matrix effect study or a qualitative post-column infusion study (5-8).
In the former, analyte is added to both extracted test samples (typically blank matrix when available) and control samples (typically solvent based, containing no matrix elements). The difference in signal between the test and control samples is expressed as a percentage and provides a quantitative expression of the extent of ion suppression or enhancement: Values <100% indicate suppression, while those >100% indicate enhancement.
The extent of a matrix effect can be calculated as non-normalized (as a ratio of peak areas) or normalized to internal standard (as a ratio of response factors, which are analyte peak areas divided by internal standard peak areas). Both provide valuable information: Non-normalized matrix effect values show the actual magnitude of ion suppression/enhancement, while the normalized values indicate how well the internal standard compensates for the matrix effect. These experiments should be performed at two concentrations expected in the patient population and with several native matrix sources, such as different patient specimens.
(Download a worked example here)
In a post-column infusion study, laboratorians infuse an analyte solution into the LC column effluent while a matrix blank sample is being analyzed (9). When blank sample matrix either is not available or is not representative of patient specimens, a solution of the isotopically labeled internal standard may be infused in place of (or in addition to) the analyte. Ion suppression (enhancement) is evaluated as the presence of negative (positive) peaks in a steady signal trace of the infused analyte or internal standard (9).
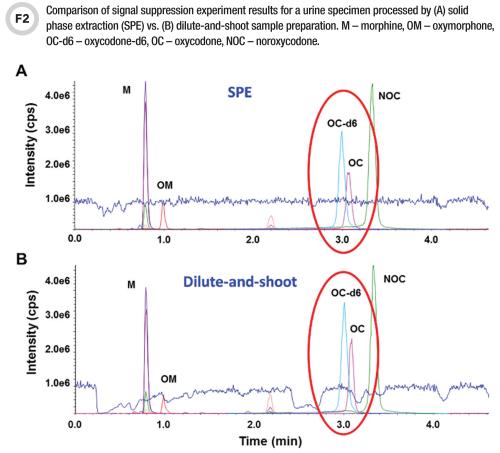
This qualitative approach enables lab personnel to visualize the position and width of matrix effects regions. This experiment is very useful during the method development stage of designing an LC gradient that will maneuver the analytes out of suppression zones—especially in the case of dilute-and-shoot methods prone to matrix effects. It is also useful for assessing extract cleanliness when determining which sample preparation method or conditions may best mitigate matrix effects (Figures 1 and 2) (3, 9).
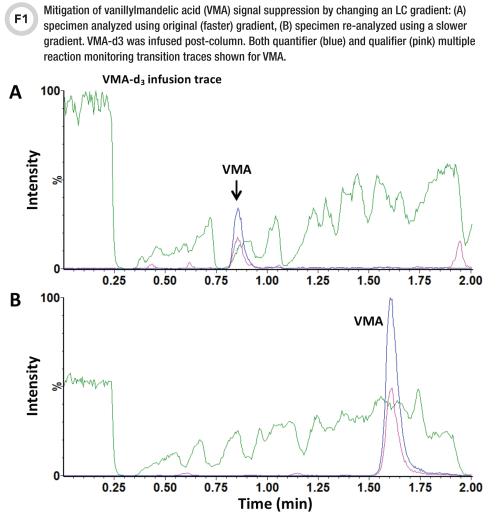
While these tactics help identify and/or reduce interferences, no method is completely immune to them. Many labs use stable isotope-labeled internal standards to increase LC-MS/MS method robustness and mitigate interference such as matrix effects. We recommend both CLSI guideline C62-A; Chapter 5.2 Internal Standard Selection and the article by Matuszewski et al. for some great tips on how to manage interferences by selecting stable-labeled internal standards while ensuring co-elution with the analytes (5, 10).
When analytes elute in a signal suppression region, compensating with an internal standard is often adequate. Problems arise, however, when the suppression severely decreases the signal-to-noise ratio of the analyte and internal standard peaks. This compromises assay performance, especially near the assay’s lower limit of quantitation (6). Another issue may stem from the internal standard not exactly co-eluting with the analyte, which sometimes happens with deuterium-labeled analogs when the deuterium atoms are in positions that impact chromatographic retention.
For example, if the internal standard elutes outside of a suppression zone while the analyte peak is partially in it, the two compounds experience a different degree of suppression, which results in inaccurate quantitation (Figure 2B). The strategies for resolving these issues include using internal standards having stable labels that do not impact chromatographic retention time (e.g., 13C, 15N, 18O) and thus better compensate for matrix effects, or the above-mentioned approaches aimed toward reducing interferences, such as LC gradient profile modifications and more selective sample preparation.
Even when labs employ the best method development strategies, they rarely are able to prevent interference completely. Consequently, they absolutely need to monitor for interference in routine testing in order to avoid reporting compromised results. The most relevant data quality metrics are ion ratios, absolute internal standard areas, and retention times, deviations in which can signal the presence of interference in either the analyte or internal standard mass chromatograms.
For additional information on quality assurance and use of these metrics, please refer to recent articles by Lynch and Zabell (11, 12).
Avoiding Unwanted Surprises
To ensure that any newly developed LC-MS/MS method is robust and provides high-quality data, we strongly advocate investigating interference by performing the experiments outlined in this article during the method development process. Labs should use as many patient specimens as practicable to ensure that they capture the biological variability of interference. Waiting until method validation to perform these experiments could result in unwanted surprises. A well-developed, validated, and controlled LC-MS/MS method will provide your laboratory with years of excellent results and is well worth the upfront effort.
- CLSI. Evaluation of matrix effects; Approved guideline – second edition. CLSI document EP14-A2.Wayne (PA): CLSI; 2005.
- CLSI. Interference testing in clinical chemistry; Approved guideline – second edition. CLSI document EP7-A2.Wayne (PA): CLSI; 2005.
- Clark ZD, Cutler JM, Pavlov IY, et al. Simple dilute-and-shoot method for urinary vanillylmandelic acid and homovanillic acid by liquid chromatography tandem mass spectrometry. Clin Chim Acta 2017;468:201–8.
- Clark ZD, Cutler JM, Frank EL. Practical LC-MS/MS method for 5-hydroxyindoleacetic acid in urine. J Appl Lab Med 2017;1:387–99.
- Matuszewski BK, Constanzer ML, Chavez-Eng CM. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem 2003;75:3019–30.
- Annesley TM. Ion suppression in mass spectrometry. Clin Chem 2003;49:1041–4.
- Bonfiglio R, King RC, Olah TV, et al. The effects of sample preparation methods on the variability of the electrospray ionization response for model drug compounds. Rapid Commun Mass Spectrom 1999;13:1175–85.
- King R, Bonfiglio R, Fernandez-Metzler C, et al. Mechanistic investigation of ionization suppression in electrospray ionization. J Am Soc Mass Spectrom 2000;11:942–50.
- Clark ZD, Strathmann FG, McMillin GA. Diluting and shooting yourself in the foot: Complications with sample-to-sample variations in signal suppression. MSACL 2013 podium presentation.
- CLSI. Liquid chromatography-mass spectrometry methods; Approved guideline. CLSI document C62-A. Wayne (PA): CLSI; 2014.
- Lynch KL. LC-MS/MS quality assurance in production: The real work begins after validation. Clinical Laboratory News 2017;43(5):28–9.
- Zabell APR, Stone J, Julian RK. Using big data for LC-MS/MS quality analysis. Clinical Laboratory News 2017;43(5):30–1.
Zlatuse Clark, PhD, is an R&D scientist at the ARUP Institute for Clinical and Experimental Pathology in Salt Lake City, Utah. +Email: [email protected]
Stephen Balloch, MChem, is a senior clinical applications scientist, Scientific Operations, at Waters Corporation in Wilmslow, U.K. +Email: [email protected]
Lisa Calton, PhD, is director of clinical and forensics, scientific operations, at Waters Corporation in Wilmslow, U.K. +Email: [email protected]
Donald Mason, MS, is global scientific affairs manager at Waters Corporation, Milford, Massachusetts. +Email: [email protected]
CLN's Focus on Mass Spectrometry is supported by Waters Corporation.
