Your patient has a dangerously low blood glucose level with no clinical explanation. The typical workup for hypoglycemia includes plasma glucose level and insulin, but the patient’s lab results for insulin are normal. This does not seem to fit the clinical picture, so you send a specimen to a reference lab for insulin testing, and the result comes back 3 times higher than the upper reference range. How is this possible? Which result is correct?
The short answer is: they both are. However, the detailed explanation is that it depends on the insulin assays being used. Different assays have varying degrees of cross-reactivity with synthetic insulin, and understanding your assay is very important in interpreting results. Synthetic insulins, such has Humalog/lispro, Novolog/aspart, Lantus/glargine, Levemir/detemir, or Apidra/glulisine are analogs of insulin that contain amino acid substitutions. These changes alter the absorption kinetics, allowing for fast or prolonged action. The location of these amino acid substitutions impacts whether or not an immunoassay can detect these analogues, and depending on the manufacturer, will provide varying results. This means that some assays will show high insulin concentrations, whereas others may show undetectable levels, which would lead to the results and confusion described above.
If your laboratory uses an assay with high cross-reactivity with insulin analogs, then your insulin tests for patients taking exogenous insulin will have high values. These results must be interpreted with caution and with knowledge of this potential cross-reactivity. Otherwise, patient, doctor, and lab could start on a lengthy, ultimately unnecessary, diagnostic odyssey. For example, an investigation of factitious hypoglycemia—deliberate use of insulin or oral hypoglycemic agents to induce low glucose levels—might ensue. Cases of intentional inappropriate insulin dosing are rare to be sure. However, if a patient does not disclose having injected synthetic insulin, another logical line of investigation would be for insulinoma, which would involve quite an extensive work-up.
If your laboratory uses an insulin assay that does not cross-react with insulin analogs, you are not home free. In this case, a patient inappropriately injecting synthetic insulin might go undetected, or be thought to be non-compliant with his or her medication. Either scenario might prompt a diagnostic odyssey to determine the cause of non-diabetic hypoglycemia, similarly resulting in unnecessary testing and treatment, or worse, a patient being treated acutely and released with no resolution.
The Role of C-Peptide
If providers suspect a patient is taking exogenous insulin, whether cross-reactivity is noted or not, pay particular attention to the insulin/C-peptide ratio. Insulin and C-peptide are co-products of proinsulin, and therefore released in the bloodstream at equimolar amounts, with insulin metabolized in the liver, and C-peptide excreted by the kidneys. The resulting insulin/C-peptide molar ratio is typically ≤ 1. However, when patients take exogenous insulin, release of C-peptide is suppressed, lowering the circulating concentration.
When the C-peptide concentration is low or undetectable but the insulin concentration is high, making the insulin:C-peptide ratio > 1, inappropriate insulin administration is a possibility. If your insulin assay does not cross-react with synthetic insulin, the C-peptide value will still be low in the case of exogenous insulin administration. A molar ratio > 1 is also useful for cases in which the insulin being administered is natural, from bovine or porcine sources, which have the same amino acid structure and typically 100% cross reactivity with insulin assays. These preparations of insulin do not contain C-peptide, so you should observe a similar abnormal molar ratio of insulin/C-peptide.
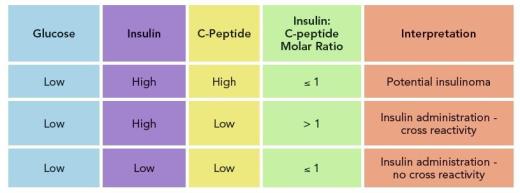
While this approach is straightforward, hospitals that lack in-house C-peptide assays may overlook it, thereby delaying treatment as they wait for C-peptide results from outside laboratories. A complete work-up including glucose, insulin, C-peptide, proinsulin, oral hypoglycemic agent screen, and insulin antibodies should be performed at the onset of hypoglycemia evaluation. Results should be considered holistically, particularly when synthetic insulin is suspected.
 Erin Kaleta, PhD, DABCC, is clinical director of the General Laboratory and Clinical Mass Spectrometry and Toxicology Laboratory at Sonora Quest Laboratories in Tempe, Arizona. +Email: [email protected]
Erin Kaleta, PhD, DABCC, is clinical director of the General Laboratory and Clinical Mass Spectrometry and Toxicology Laboratory at Sonora Quest Laboratories in Tempe, Arizona. +Email: [email protected]