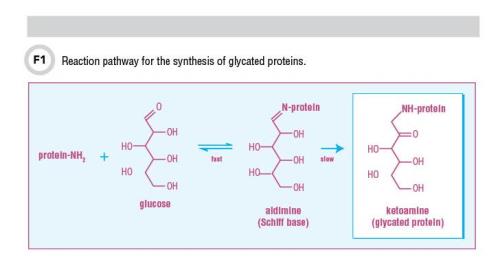
Measuring glycated proteins in people with diabetes has been a mainstay for assessing both glycemic control and risk for diabetes complications. Glycated proteins are produced by the relatively quick and reversible two-step nonenzymatic reaction called the Maillard early-phase reaction, in which the free amino group of a protein and the aldehyde group of glucose form a Schiff base or aldimine. This aldimine then undergoes a slowly progressing and almost irreversible Amadori rearrangement to become a stable ketoamine or Amadori product that is a glycated protein. This reaction pathway for the synthesis of glycated proteins is shown in Figure 1. Since the rate of glycated protein production is proportional to the plasma glucose level as well as the half-life of the protein, glycation rises in patients with diabetes due to increased levels of glucose. Some of these glycated proteins can be used as markers of glycemic control and others may also be directly involved in the onset and development of chronic diabetic complications.
The Role of HbA1c
Glycated hemoglobin (HbA1c) is the general term to describe all hemoglobin bound to glucose following the reaction pathway shown in Figure 1. Glucose can be bound to several different sites on hemoglobin including the N-terminus of the beta chain (HbA1c), which is the most predominate glycated species, or the alpha chain as well as epsilon amino groups of lysine residues. In the case of HbA1c, since the protein resides in the erythrocytes, glycation is related to the lifespan of erythrocytes. HbA1c provides an estimate of mean blood glucose over the lifespan of the erythrocyte (3–4 months). Although the first commercial HbA1c test became available in the late 1970s, the clinical usefulness of HbA1c did not become clear until 1993, when results from the landmark Diabetes Control and Complications Trial (DCCT) were reported (1). This trial confirmed the relationship between HbA1c and diabetes complications and set the stage for specific treatment goals for people with diabetes.
Based on DCCT results, the American Diabetes Association, as well as other worldwide diabetes organizations, recommend routinely measuring HbA1c with specific HbA1c targets to monitor glycemic control in diabetes. The initial lack of standardization hindered the use of these targets in clinical practice. Thus, the National Glycohemoglobin Standardization Program (NGSP) was initiated in 1996 with the goal of standardizing reported results to the DCCT reference regardless of the glycated species measured (HbA1c or total glycated hemoglobin). Efforts by the NGSP (www.ngsp.org) to standardize HbA1c testing have been highly successful, resulting in much improvement in the comparability of results. It was mainly for this reason that, in 2010, the American Diabetes Association, followed by other worldwide clinical organizations, recommended HbA1c for use in diagnosing diabetes.
As patients with diabetes are generally assessed 2–4 times per year, HbA1c provides an estimate of mean glucose during the time between doctor visits. Although HbA1c is an extremely useful measure of glycemic control in most people with diabetes, the test has certain limitations. Method-specific interferences from hemoglobin variants, conditions that alter erythrocyte lifespan such as hemolytic anemia, severe iron deficiency anemia, and other factors can affect HbA1c results.
Glycated Serum Proteins and Glycated Albumin
Serum proteins, including albumin, have a shorter half-life compared to the lifespan of erythrocytes. Consequently, glycated serum proteins (GSPs), also called fructosamines, represent mean glycemia over the prior 2–4 weeks. GSP measurements at this time are not generally recommended as a substitute for HbA1c, in part due to the lack of assay standardization and specific treatment cutoffs, and the shortened timeframe in relation to mean glycemia. However, these measures may be useful when HbA1c cannot provide an accurate assessment or when a shorter timeframe of mean glycemia would be useful.
A method for measuring GSP was developed in the 1980s based on the ability of serum fructosamines to reduce nitroblue tetrazolium (NBT) and change the dye’s absorbance (2). This assay soon was commercialized and came to be known as the “fructosamine assay.” More recently, enzymatic assays for measuring fructosamines have been described. Glycated albumin (GA) comprises nearly all of GSP, since albumin is the most prevalent protein in serum. An assay method for measuring GA also has been described (3).
Initially marketed as a substitute for HbA1c, the NBT fructosamine assay was not well accepted. Most clinical organizations never recommended it as an alternative to HbA1c, and expert opinion remained divided on its clinical relevance. Since then, many improvements have been made to the assay, such as better specificity with less susceptibility to interferences, better calibration material, and other changes. Even with these enhancements, the issue of whether this measurement needs to be adjusted for the total amount of protein in each sample still has not been resolved satisfactorily. Early studies showed that the measurement was independent of protein concentration as long as the protein concentration was within the reference range. However, others found statistically significant relationships between fructosamine and protein concentrations and recommended that results be corrected for protein concentration (4).
Recently, researchers have focused increasing attention on the use of GA as an intermediate indicator of glycemic control. Though several methods have been described over the years, the one given the most attention has been the enzymatic assay described by Kohzuma et al. using an albumin-specific proteinase and reporting results as percentage of total albumin (3). This assay was later optimized and several studies have documented its performance and clinical usefulness. Research showed it to be linear over the clinical assay range (3.2–68.1%) and demonstrated within- and between-run coefficients of variation of less than 1%. In addition, a reference range in an American population has been reported (5).
Several recent studies have examined the association of GA with diabetes complications. One study measured GA in a subgroup of DCCT subjects using the enzymatic assay, and, as expected, HbA1c and GA were highly correlated. The researchers also found that GA paralleled HbA1c over the course of the study. In addition, both HbA1c and GA were associated strongly with progression of retinopathy and nephropathy (6). Another cross-sectional study of participants from the Atherosclerosis Risk in Communities Study compared HbA1c, GA, and fructosamine to risk of chronic kidney disease (CKD), retinopathy, and coronary heart disease. This investigation found different associations of each glycemic marker with demographic and clinical characteristics: GA and GSP were more strongly affected by postprandial glycemic excursions compared with HbA1c, but all three were similarly positively associated with prevalent CKD and retinopathy (7).
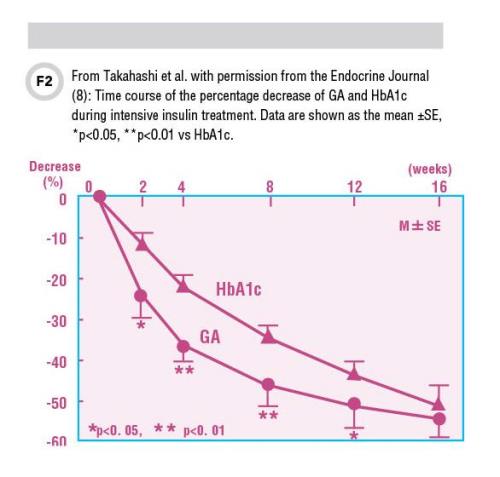
In some cases, when clinicians need to follow patients over the short term to monitor the effects of intensive therapy, GA or fructosamine may be more useful than HbA1c. Figure 2 shows the profile of percentage decrease of GA and HbA1c during 16 weeks of intensive insulin therapy in 28 previously untreated patients with Type 2 diabetes. In this study, GA showed a significantly larger decrease than HbA1c in as early as 2 weeks. The difference persisted until week 16, when the percentage decrease in both GA and HbA1c reached a similar level (8).
While HbA1c remains the best measure of glycemic control in nearly all patients with diabetes—and the intermediate-term measures of glycemic control still have not been widely accepted and generally do not appear in most clinical diabetes recommendations—HbA1c is not the most appropriate marker of glycemic control in all circumstances. One of the most important of these situations is in CKD leading to end-stage renal disease or kidney failure.
Diabetes is the leading cause of CKD, and patients with diabetes on dialysis have very poor survival. It has been difficult to test the impact of aggressive glycemic control in dialysis patients with diabetes in part due to concerns about the validity of HbA1c in predicting outcomes. This is despite continued recommendations by the National Kidney Foundation (NKF) to use the same target HbA1c irrespective of the presence or absence of CKD (9). However, the NKF does mention that there is “some degree of inaccuracy of the HbA1c measurement” in patients with “progressive kidney disease.”
Many have observed that HbA1c is falsely lowered in CKD due to shortened erythrocyte lifespan as well as an increased ratio of immature erythrocytes following the administration of erythropoietin. Studies have found that in comparison to those without renal disease, GA concentrations were significantly higher in both Japanese and American diabetic subjects with renal disease, while HbA1c tended to be lower (10, 11). In addition, HbA1c was positively associated with hemoglobin concentration and negatively associated with the erythropoietin dose in hemodialysis patients, while these factors and serum albumin did not significantly impact GA levels (11). In another study of patients on both hemodialysis and peritoneal dialysis, HbA1c significantly underestimated glycemic control relative to GA in both patient treatment groups (12).
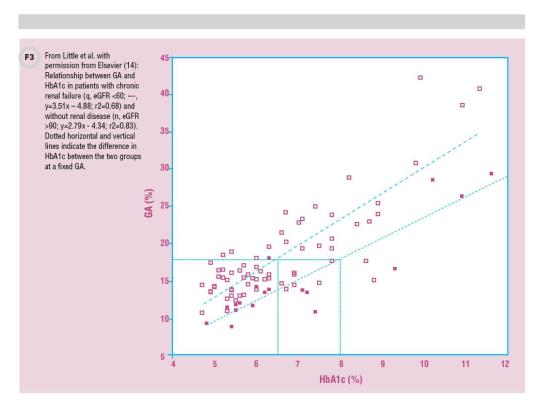
Similarly, a study of patients with advanced CKD (stage 3 or stage 4) but not on dialysis found HbA1c falsely reduced compared to GA (13). Finally, a study in 2013 demonstrated that while modern HbA1c assays did not have analytical interference from increased carbamylated Hb, there was indeed interference due to changes in average erythrocyte lifespan with renal failure. Consistent with other studies, HbA1c results were lowered by approximately 1.5% at critical treatment levels in patients with renal failure (14) (Figure 3).
Unfortunately, various factors also interfere with GA. In disorders influencing albumin metabolism, serum GA may not accurately reflect glycemic control. These conditions are listed in Table 1. For example, very high levels of proteinuria in nephrotic syndrome have been shown to decrease GA independent of glycemia in diabetic patients with CKD (15). Body mass also plays a role. A significant inverse correlation between body mass index and GA in non-diabetic subjects, independent of plasma glucose levels has been reported (16), and in fact, obesity may artificially lower GA due to increased inflammation with concomitant increased serum albumin turnover (15).
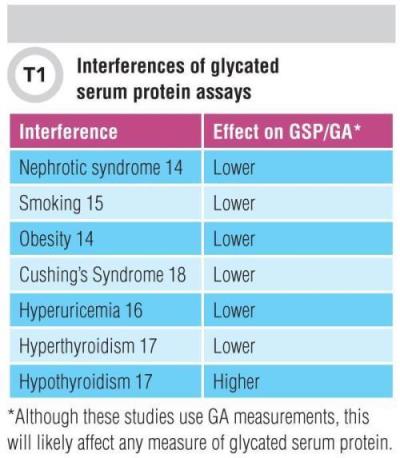
In smokers with increased levels of C-reactive protein measured by high-sensitivity assays, (17) and in men with hyperuricemia (18), serum GA was also artificially low. The theory is that inflammation-induced acceleration of albumin metabolism may be involved in all of these situations. Thyroid hormone levels also are a source of interference, sometimes inversely associated with GA levels. This means that laboratories should use caution when interpreting GA levels in patients with thyroid dysfunction (19). GA also is lower in patients with Cushing’s syndrome, likely due to high glucocorticoids promoting protein catabolism including serum albumin (20). While we have described GA-related interferences, it is reasonable to assume that these situations would also influence interpretation of any measure of serum protein glycation, e.g., fructosamine.
Considerations in Choosing an Assay
Clinical laboratory professionals should consider several issues when deciding whether to measure either fructosamines (GSP) or GA. First, given the lack of standardization and specific clinical guidelines for using these assays, laboratorians themselves should compare the assay methods and how results would be interpreted. Some of the current assays for GSP/fructosamine are Food and Drug Administration (FDA)-approved and have documented good performance (7). In contrast, although the performance and clinical usefulness of the enzymatic GA assay has been documented very well, it is not yet FDA-approved. Clinical diabetes organizations in the U.S. do not specifically recommend use of these intermediate glycemic markers; however, they are used routinely in some other countries such as Japan. There are situations when measuring an intermediate-term marker is warranted, but as with HbA1c, healthcare providers need to be aware that there are situations in which these assays can give falsely low or high results relative to glycemic control.
Randie R. Little, PhD, is the National Glycohemoglobin Standardization Program Network Coordinator and the co-director of the diabetes diagnostic laboratory at the University of Missouri School of Medicine in Columbia. Email: [email protected]
References
1. New Engl J Med 1993;329:977–86.
2. Diabetes Care 1993;16:1313–4.
3. Clin Chim Acta 2002;324:61–71.
4. Diabetes Care 2004;27:1761–73.
5. J Diabetes Sci Technol 2011;5:1455–62.
6. Diabetes 2014;63:282–90.
7. Diabetes Care 2011;34:960–7.
8. Endocr J 2007;54:139–44.
9. National Kidney Foundation KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 update. http://www.ajkd.org/article/S0272-6386(12)00957-2/pdf (Accessed May 2016).
10. J Am Soc Nephrol 2007;18:896–903.
11. Kidney Int 2008;73:1062–8.
12. Peritoneal Dialysis International 2010;30:72–9.
13. Am J Nephrol 2010;31:375–9.
14. Clin Chim Acta 2013;418:73–6.
15. Intern Med 2010;50:23–9.
16. Clin Chim Acta 2007;378:48–52.
17. Acta Diabetologica 2009;46:141–4.
18. Acta Diabetologica 2010;47:173–7.
19. Diabetes Res Clin Pract 2009;84:163–7.
20. Clin Chim Acta 2013;424:164–7.