Next generation technologies (NGT) deliver huge improvements in cost efficiency, accuracy, robustness, and in the amount of information they provide. Microarrays, high-throughput sequencing platforms, digital droplet PCR, and other technologies all offer unique combinations of desirable performance.
As stronger evidence of genetic testing’s clinical utility influences patterns of patient care, demand for NGT testing is increasing. This presents several challenges to clinical laboratories, including increased urgency, clinical importance, and breadth of application in molecular oncology, as well as more integration of genetic tests into synoptic reporting. Laboratories need to add NGT-based protocols while still providing old tests, and the pace of change is increasing.What follows is one viewpoint on the major challenges in adopting NGTs into diagnostic molecular oncology service.
Choosing a Platform
Instrument selection is a critical decision that has to align with intended test applications, sequencing chemistries, and analytical software. Although multiple platforms are available, a mainstream standard has not emerged. Depending on their goals, laboratories might set up NGTs for improved accuracy of mutation detection, massively higher sequencing capacity per test, massively more targets combined in one test (multiplexing), greater range in sequencing read length, much lower cost per base pair assessed, and economy of specimen volume.
When high-throughput instruments first made their appearance, laboratories paid more attention to the accuracy of base-reading: Less accurate sequencing meant more data cleaning and resequencing (1). Now, new instrument designs have narrowed the differences, and test chemistry can have a comparatively large impact on analytical accuracy (Figure 1). The robustness of technical performance can also vary significantly depending upon specimen type. For example, LifeTechnologies’ sequencing platforms appear to be comparatively more tolerant of low DNA quality and concentration, which is an important consideration for fixed and processed tissues.
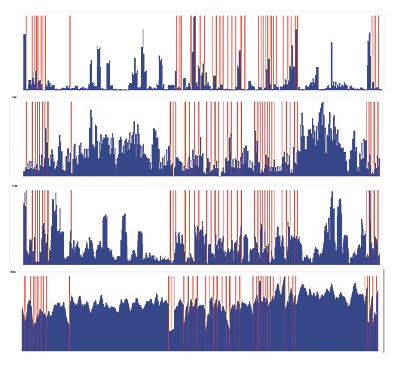
Figure 1 Comparison of Sequencing Chemistries
Sequence pile-ups of the same target sequence (2 large genes), all performed on the same analytical instrument. Results from 4 different chemistries, as designed and supplied by reagent manufacturers prior to optimization in the laboratory. Red lines represent limits of exons. Height of blue columns proportional to depth of coverage. In this case, the intent of the test design was to provide high depth of coverage so that reflex Sanger sequencing would not be necessary. Courtesy B. Sadikovic, U. of Western Ontario.
In addition, batching, robotics, workload volume patterns, maintenance contracts, software licenses, and platform lifetime affect the cost per analyte and per specimen considerably. Royalties and reagent contracts also factor into the cost of operating NGT: In some applications, fees for intellectual property can represent more than 50% of the bench cost of performing a given test, and increase substantially without warning.
Laboratories must also deal with the problem of obsolescence. Investing in a new platform brings the angst of knowing that better machines and chemistries are just around the corner. Laboratories are buying bigger pieces of equipment with shorter service lives. Before NGTs, major instruments could confidently be expected to remain current for at least 6 to 8 years. Now, a major instrument is obsolete much sooner, often within 2 to 3 years. This means that keeping it in service might cost more than investing in a new platform. Lease-purchase arrangements help mitigate year-to-year fluctuations in capital equipment costs, and maximize the value of old equipment at resale.
One Size Still Does Not Fit All
Laboratories face numerous technical considerations to optimize sequencing protocols, but the test has to be matched to the performance criteria needed for the clinical indication (2). For example, measuring response to treatment depends first upon the diagnostic recognition of mutation(s) in the tumor clone; the marker(s) then have to be quantifiable and indicative of tumor volume throughout the course of disease (Table 1).
As a result, diagnostic tests need to cover many different potential mutations, yet accurately identify any clinically relevant mutations actually present. On the other hand, tests for residual disease need to provide standardized, sensitive, and accurate quantification of a selected marker mutation against the normal background. A diagnostic panel might need 1% to 3% sensitivity across many different mutations. But quantifying early response to induction—and later assessment of minimal residual disease—needs a test that is reliably accurate to the 10-4 or 10-5 range for a specific analyte.
Covering all types of mutations in one diagnostic test is not yet possible. For example, subtyping of acute myeloid leukemia is both old school (karyotype, fluorescent in situ hybridization, and/or PCR-based or array-based testing for fusion rearrangements, deletions, and segmental gains) and new school (NGT-based panel testing for molecular mutations).
Chemistries that cover both structural variants and copy number variants are not yet in general use, but the advantages of NGTs compared to traditional methods are becoming clearer, such as in colorectal cancer (3). Researchers are also using cell-free DNA (cfDNA) to quantify residual disease and detect resistance mutations (4). Once a clinically significant clone is identified, enrichment techniques help enable extremely sensitive quantification of residual disease (5).
Validation and Quality Assurance
Beyond choosing a platform, two distinct challenges arise in bringing NGTs into the lab. The first is assembling the resources for validation and quality assurance. The second is keeping tests up-to-date as new analytes are needed. Even if a given test chemistry has the flexibility to add analytes without revalidating the entire panel, keeping up with clinical advances is a constant priority.
Due to their throughput and multiplexing capacities, NGT platforms typically require considerable upfront investment to adopt, and training staff to perform testing takes even more time. Proper validation is harder to document: Assembling positive controls, documenting test performance criteria, developing quality assurance protocols, and conducting proficiency testing are all demanding. Labs meet these challenges in different ways. Laboratory-developed tests (LDTs) allow self-determined choice in design, innovation, and control of the test protocol, but can be very expensive to set up.
Food and Drug Administration (FDA)-approved methods are attractive but not always an option. More FDA-approved methods will be marketed, but FDA approval itself brings other trade-offs. There is a cost premium compared to LDTs, and the test methodologies are locked down and not modifiable. This is particularly frustrating for NGTs, which have the specific attraction of extensive multiplexing capacity and accommodating new analytes.
IT and the Evolution of Molecular Oncology Reporting Standards
The options for information technology (IT) pipelines for NGTs are improving rapidly. At the same time, recent studies still show significant inconsistencies and lack of reproducibility when it comes to interpreting variants in array comparative genomic hybridization, panel testing, tumor expression profiling, and tumor genome sequencing. It can be difficult to duplicate published performances in clinical studies because of a lack of sufficient information about the protocol (chemistry) and software. Building bioinformatics capacity is a key requirement, yet skilled people are in short supply and the qualifications needed to work as a bioinformatician in a clinical service are not yet clearly defined.
Tumor biology brings another level of complexity. Bioinformatic analysis must distinguish tumor-specific variants from genomic variants. Sequencing of paired normal tissue is often performed as a control, but virtual normal controls may have intriguing advantages (6). One of the biggest challenges is to reproducibly interpret the clinical significance of interactions between different mutations, even with commonly known, well-defined mutations (7). For multiple analyte panels, such as predictive testing for breast cancer, only the performance of the whole panel in a population of patients can be compared; individual patients may be scored into different risk categories by different tests, all for the same test indication.
In large scale sequencing of tumor genomes, which types of mutations are most informative in detecting, quantifying, and predicting the behavior of the tumor over time? The amount and complexity of mutation varies considerably across different tumor types, and while some mutations are more common, stable, and clinically informative than others, the utility of a given tumor marker varies in different clinical situations. And, for a given tumor, treatment effect and metastasis leads to retesting for changes in drug sensitivities.
These complexities mean that IT must be designed into the process from the beginning. Like robotics, IT represents a major ancillary decision. One approach many labs choose is licensed technologies with shared databases that are updated in real time. These are attractive, despite their cost and licensing fees. New tests that incorporate proprietary IT with NGT platforms link the genetic signatures of tumors to clinically significant considerations like tumor classification, recommended methodologies for monitoring response, predicted drug sensitivities, eligible clinical trials, and prognostic classifications. In-house development of such solutions will be difficult, so licensing platforms from commercial partners is more likely to be the norm.
The Commercial Value of Health Records and Test Data
The future of cancer management likely rests on large-scale databases that link hereditary and somatic tumor testing with clinical outcomes. Multiple centers have such large studies underway, and data extraction and analysis is providing increasingly refined interpretations of clinical significance.
Extracting health outcomes to correlate with molecular test results is commercially valuable, as the pharmaceutical, insurance, and healthcare sectors focus on companion diagnostics, precision medicine, and evidence-based health technology assessment. Laboratories that can develop tests based on large-scale integration of test results to clinical utility will have an advantage.
NGTs do offer opportunities for net reductions in the cost of healthcare. But the lag between availability of a test and peer-evaluated demonstration of clinical utility can be considerable. Technical developments arise faster than evidence of clinical utility. For example, immunohistochemistry, estrogen receptor/progesterone receptor status, HER2/neu, and histology are still the major pathological criteria for prognostic evaluation of breast cancer at diagnosis, even though multiple analyte tumor profiling has been described for more than 15 years. Healthcare systems need a more concerted assessment of clinical utility if they are to take advantage of the promises of NGTs in cancer care.
Disruptive Advances
Without a doubt, "disruptive" is an appropriate buzzword in molecular oncology, and new technical advances are about to change how, where, and for whom testing is performed.
• Predictive Testing
Besides cost per analyte, one of the drivers for taking up new technologies is that they enable multiplexing many more analytes with less biopsy material. Single-analyte sequential testing for epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase, and other targets on small biopsies is not sustainable when many more analytes are needed, and even now, a significant proportion of test requests cannot be completed due to lack of suitable biopsy material. Large panels incorporating all the mutations needed to cover multiple tumor types are replacing individual tests in companion diagnostics.
• Cell-Free Tumor DNA
Challenges of cfDNA include standardizing the collection and processing methodologies, timing sampling to minimize the effect of therapeutic toxicity on analytical accuracy, and identifying the most informative sample (DNA, RNA, or protein). But for more and more tumor types, it will be possible to differentiate benign versus malignant lesions, perform molecular subtyping, predict response, monitor treatment, or screen for early detection—all without a surgical biopsy.
cfDNA technologies can also be integrated into core laboratory instrumentation. For example, blood-based EGFR analysis for lung cancer is being developed on the Roche cobas 4800 platform, which will be a significant change from the current standard of testing based upon single tests of DNA extracted from formalin-fixed, paraffin-embedded sections selected by a pathologist (8).
• Whole Genome and Whole Exome Sequencing
Whole genome and whole exome tumor sequencing approaches provide a wealth of biologically important information, and will replace individual or multiple gene test panels as the technical cost of sequencing declines and interpretive accuracy improves (9). Laboratories can apply informatics selectively or broadly to extract much more information at relatively little increase in cost, and the interpretation of individual analytes will be improved by the context of the whole sequence.
• Minimal Residual Disease Testing
Massive resequencing and enrichment techniques can be used to detect minimal residual disease, and will provide an alternative to flow cytometry as costs decline. The challenge is to develop robust analytical platforms that can reliably produce results in a high proportion of patients with a given tumor type, despite using post-treatment specimens with therapy-induced degradation, and a very low proportion of target (tumor) sequence to benign background sequence.
The tumor markers should remain informative for the burden of disease despite clonal evolution over the course of multiple samples taken during progression of the clinical course and treatment. Quantification needs to be accurate and sensitive down to the 10-5 range, and cost competitive with flow cytometry.
• Point-of-Care Test Methodologies
Small, rapid, cheap, and single use point-of-care (POC) sequencing devices are coming. Some can multiplex with analytical times as short as 20 minutes. Accurate and timely testing will be possible in places like pharmacies, oncology clinics, patient service centers, and outreach programs. Whether physicians will trust and act on POC results alone, or will require confirmation by traditional laboratory-based testing, remains to be seen. However, in the simplest type of application, such as a patient known to have a particular mutation, the advantages of POC-based testing to quantify residual tumor burden are clear.
Conclusion
Molecular oncology is moving rapidly from an esoteric niche of diagnostics to a mainstream, required component of integrated clinical laboratory services. While NGTs are markedly reducing the cost per analyte and per specimen, and will certainly broaden the scope and volume of testing performed, the resources required to choose, install, and validate these new technologies are daunting for smaller labs. More rapid obsolescence and increased regulatory scrutiny for LDTs also present significant challenges. Aligning test capacity with approved clinical indications will require careful and constant attention to ensure competitiveness.
References
1. Liu L, Li Y, Li S, et al. Comparison of next-generation sequencing systems. J Biomed Biotechnol 2012; doi:10.1155/2012/251364.
2. Brownstein CA, Beggs AH, Homer N, et al. An international effort towards developing standards for best practices in analysis, interpretation and reporting of clinical genome sequencing results in the CLARITY Challenge. Genome Biol 2014;15:R53.
3. Haley L, Tseng LH, Zheng G, et al. Performance characteristics of next-generation sequencing in clinical mutation detection of colorectal cancers. [Epub ahead of print] Modern Pathol July 31, 2015 as doi:10.1038/modpathol.2015.86.
4. Butler TM, Johnson-Camacho K, Peto M, et al. Exome sequencing of cell-free DNA from metastatic cancer patients identifies clinically actionable mutations distinct from primary disease. PLoS One 2015;10:e0136407.
5. Castellanos-Rizaldos E, Milbury CA, Guha M, et al. COLD-PCR enriches low-level variant DNA sequences and increases the sensitivity of genetic testing. Methods Mol Biol 2014;1102:623–39.
6. Hiltemann S, Jenster G, Trapman J, et al. Discriminating somatic and germline mutations in tumor DNA samples without matching normals. Genome Res 2015;25:1382–90.
7. Lammers PE, Lovly CM, Horn L. A patient with metastatic lung adenocarcinoma harboring concurrent EGFR L858R, EGFR germline T790M, and PIK3CA mutations: The challenge of interpreting results of comprehensive mutational testing in lung cancer. J Natl Compr Canc Netw 2015;12:6–11.
8. Weber B, Meldgaard P, Hager H, et al. Detection of EGFR mutations in plasma and biopsies from non-small cell lung cancer patients by allele-specific PCR assays. BMC Cancer 2014;14:294.
9. Vogelstein B, Papadopoulos N, Velculescu VE, et al. Cancer genome landscapes. Science 2013;339:1546–58.
10. Heitzer E, Auer M, Gasch C, et al. Complex tumor genomes inferred from single circulating tumor cells by array-CGH and next-generation sequencing. Cancer Res 2013;73:2965–75.
11. Healy B. BRCA genes — Bookmaking, fortunetelling, and medical care. N Engl J Med 1997;336:1448–9.
Ronald F. Carter, PhD, DVM, is a professor emeritus in the department of pathology and molecular medicine at McMaster University in Hamilton, Ontario. He is also the program director of Lifelabs Genetics, Toronto. +Email: [email protected]