Assessing serum biological markers is an essential component of detecting and monitoring multiple myeloma disease progression. Multiple myeloma is the second most common blood malignancy, and represents approximately 1% of all cancers with a disease burden of approximately 120,000 new cases worldwide per year. Multiple myeloma is typically characterized by the observation of monoclonal plasma cells, the presence of circulating monoclonal protein immunoglobulin, and related organ damage. Evidence of organ damage resulting from multiple myeloma clonal plasma cell proliferative disorder includes the CRAB criteria of hypercalcemia, renal insufficiency, anemia, and bone lesions. Improvements in treatment, diagnosis, and monitoring are continually leading to better survival rates as evidenced by a higher 5-year survival rate of 35% in 2005–2009 versus 10% in the 1970s. This review will focus on circulating protein markers of multiple myeloma, although rapid progress is being made in genetic analysis of this disorder.
Current Guidelines and Diagnostic Criteria
According to the 2014 International Myeloma Working Group (IMWG) guideline on diagnosis of multiple myeloma and smoldering multiple myeloma, the diagnostic criteria for multiple myeloma includes observation of monoclonal plasma cells, monoclonal protein (in most cases), and related organ damage. Monoclonal cell proliferation is observed by means of bone marrow aspiration and/or biopsy. Bone lesions are detected by radiographic techniques such as a traditional whole body skeletal bone survey or magnetic resonance imaging (MRI), computed emission tomography, or positron emission tomography.
The IMWG guidelines also consider the role of circulating protein markers, measured by protein electrophoresis and immunofixation electrophoresis, determination of total serum immunoglobulin concentrations, and serum free light chain (FLC) analysis with derived kappa/lambda ratios. Other analytes of importance in the work-up of suspected or confirmed multiple myeloma include complete blood count, albumin, Beta2 microglobulin, calcium, creatinine, lactate dehydrogenase, total serum protein, liver function tests, C-reactive protein, and vitamin D.
The guidelines define multiple myeloma as >10% clonal bone marrow cells or plasmacytoma, with clinical end organ damage such as CRAB, or the presence of at least one of the following: >60% clonal bone marrow cells; an involved/uninvolved serum FLC ratio >100; or more than one focal lesion in MRI studies. Once diagnosis is established, guidelines for risk stratification in the international staging system use a combination of increasing Beta2 microglobulin concentrations and decreasing albumin measurements to indicate increased risk stratification.
Monoclonal Gammopathy of Undetermined Significance
Multiple myeloma is thought to be preceded almost universally by an asymptomatic state of monoclonal gammopathy of undetermined significance (MGUS), in which some monoclonal protein (<30 g/L) and plasma cells are present (<10%), but with an absence of the CRAB attributes or other clinical symptoms. While this premalignant state is relatively prevalent, occurring in about 4% of individuals older than age 50, relatively few proceed to frank multiple myeloma. The rate of conversion from MGUS to multiple myeloma is approximately 0.5–1.0% per year.
Smoldering multiple myeloma is an intermediate stage between MGUS and frank disease characterized by higher levels of plasma cells (10–60%) and monoclonal protein (>30 g/L), yet without further clinical symptoms. Smoldering multiple myeloma patients exhibit a much higher rate of conversion to frank disease—10% in 5 years—compared to those with MGUS. Progression rates among those with MGUS or smoldering multiple myeloma appear to be variable among different individuals, and work continues on prognostic markers to gauge relative risk of progression.
Protein Biomarkers
Immunoglobulin FLCs were one of the first tumor markers for multiple myeloma. Since their discovery, a great deal of progress has been made in identifying new protein markers for detecting, monitoring, and determining prognosis for multiple myeloma. Ways to detect these new markers include serum protein electrophoresis and determination of serum FLC by nephelometry and other methods. Also important are assays for light chain/heavy chain-specific intact immunoglobulin subsets: IgGκ, IgGλ, IgAκ, and IgAλ (heavy/light chain immunoassays or HLC).
Serum Protein Electrophoresis and Immunofixation Electrophoresis
Monoclonal proteins are routinely visualized by serum protein electrophoresis (SPEP) and/or immunofixation electrophoresis (IFE) (See Figures 1 and 2). IFE and SPEP can also be performed on urine to look for the presence of Bence Jones proteins and are often designated as UIFE and UPEP analysis. However, urinalysis protein strips are relatively insensitive to Bence Jones proteins, and absence of elevated urine protein does not exclude multiple myeloma.
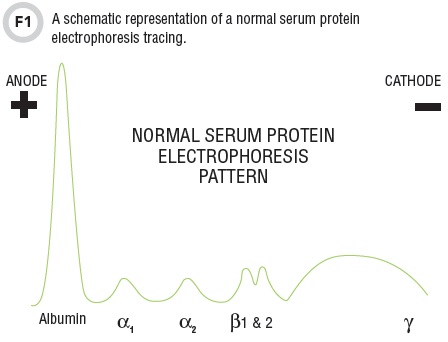
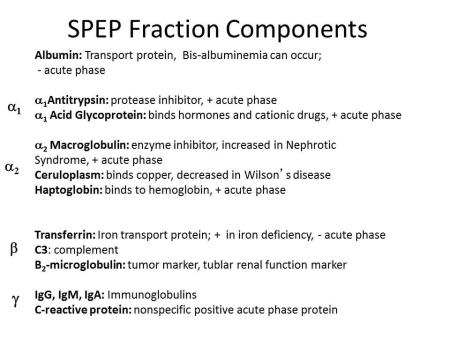
In traditional SPEP, sera is separated into major serum fractions on agarose or cellular acetate separation beds and visualized by staining and densitometry. The net negative charge of albumin migration yields a distinct strong band migrating closest to the positive anode (note that the charge of the anode and cathode are reversed in comparison to traditional chemistry and physics designations). From anode to cathode are the migrations of albumin, alpha1 (includes alpha-1-acid glycoprotein and alpha-1 antitrypsin), alpha 2 (includes alpha 2-macroglobulin and haptoglobin), beta 1/2 (includes transferrin and C3), and gamma region (predominantly consists of immunoglobulins), all of which are illustrated in Table 1.
Protein patterns can be altered in the presence of monoclonal protein (M-spike present), AAT deficiency (reduction in alpha-1), as well as nephrotic, acute phase, or inflammatory syndromes, which can result in changes in multiple fractions. Additionally, capillary zone electrophoresis multichannel techniques have been introduced that monitor migration of serum proteins by recording optical absorption at 210 nm in an optical window in the capillary.
All SPEP methods generally allow for quantitation of monoclonal protein fractions. Total nephelometric immunoglobulin measurements can be used to monitor disease progression at high concentrations; however, at lower monoclonal concentrations their utility is compromised by the presence of polyclonal immunoglobulins.
Immunofixation electrophoresis can be roughly described as a qualitative method for visualizing classes of monoclonal immunoglobulins and is generally more sensitive than SPEP. In this method, serum immunoglobulins are separated by electrophoresis, and different lanes are exposed to antibodies against immunoglobulin classes which bind and fix the immunoglobulin bands for staining and visualization. Overall, IgG is the most prevalent heavy chain associated with multiple myeloma clones (52%), followed by IgA (21%). Approximately 20% of myeloma clones express FLC only. The less common IgD monoclonal protein heavy chain is observed in 2% of myeloma clones and IgM heavy is found in 0.5% of myeloma clones.
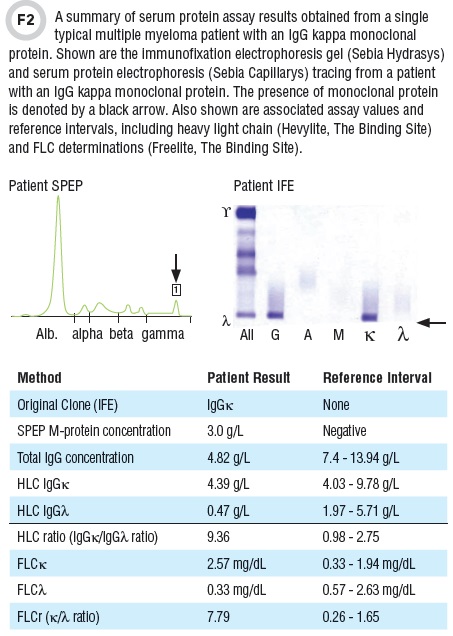
Notably, approximately 2% of myelomas are biclonal and 2% do not exhibit appreciable monoclonal protein by SPEP or IFE and are considered non-secretory. IgE monoclonal proteins are extremely rare. Observation of FLC in IFE such as that shown in Figure 2 must be checked using antisera against IgD and IgE to ensure that there is not an unobserved associated heavy chain present in the initial IFE electrophoresis analysis. The technique of immunosubtraction or selective removal of immunoglobulin subtypes by antibody capture and subsequent comparison of SPEP patterns has been utilized successfully to type monoclonal protein but may be less sensitive than IFE.
Both SPEP and IFE analysis of monoclonal proteins have notable limitations. IFE requires experienced analysis and often exhibits immunoglobulin restrictions that mimic monoclonal protein or broad nonspecific areas of immunoglobulin migration and impede identification of monoclonal protein. Without corroborating biochemical or clinical evidence, care must be taken not to overcall as definitive monoclonal protein observed bands of moderate to weak intensity.
There are also significant challenges with determining monoclonal protein concentrations by SPEP. Among these are overlapping non-immunoglobulin proteins in serum protein electrophoresis, particularly in the case of monoclonal proteins, which run more anodal.
Another limitation is that overlap of monoclonal proteins can make quantification by electrophoresis challenging and subjective. IgG clones typically migrate in the gamma region, giving rise to the Gaussian gamma region pattern seen in normal SPEP analysis. However, IgA and IgM monoclonal proteins often run more anodal than IgG, overlapping other serum proteins (nonspecific IgA clones give rise to the beta bridging pattern in cirrhosis). The presence of non-tumor polyclonal immunoglobulins (Ig’κ and Ig’λ) of a given isotype potentially obscures clinical assessment of low concentrations of monoclonal protein. SPEP determinations can exhibit relatively high imprecision at concentrations below 10.0 g/L.
Care must be taken not to introduce plasma into protein electrophoresis, as the presence of fibrinogen can give a false band in the beta gamma region which can be reversed upon addition of thrombin or ethanol. Additionally, the presence of non-tumor polyclonal immunoglobulins potentially obscures clinical assessment of low concentrations of monoclonal protein, and albumin determinations may be significantly higher relative to chemical dye binding methods in the presence of substantial monoclonal protein.
Immunoassays
Although monoclonal serum FLC can be quantified by SPEP in some cases, the use of a serum FLC nephelometric or turbidimetric polyclonal immunoassay has become standard practice for measuring free kappa (κ) and free lambda (λ) light chains in monoclonal gammopathies and other patient specimens. Alternative monoclonal FLC assays are also becoming available. FLC assays are designed to be specific to free immunoglobulin light chains and not react with light chains bound to heavy chains. In addition to high sensitivity and specificity, another advantage of serum FLC assays is that they can be used to calculate κ/λ ratios. Furthermore, the short half life of FLCs (2–6 hours) and relative in vitro stability (several weeks in refrigerated serum) also are useful analytical characteristics.
While current serum FLC assays are automated and have well-established reference intervals, they also have some limitations. These include potential non-linear dilution of monoclonal FLCs in the assay, and the possibility of antigen excess of the nephelometric assay.
Antigen excess can lead to potentially disastrous and misleading analytical reduction of measured FLC concentrations even down to relatively normal concentrations when high concentrations actually exist. In cases of possible antigen excess where there is potential for falsely low results, such as on a new patient—especially in the concurrent presence of substantial FLC by IFE, or high concentrations of associated total heavy chain—further dilution of the specimen should be considered.
Serum FLC Ratios
Since introduction of the first commercially available serum FLC assay, the clinical value of these tests has been well demonstrated. Most myeloma symptomatic patients (95%) exhibit abnormal κ/λ FLC ratios (FLCr). The value of serum FLC in detecting and screening for disease has been consistently verified, such that FLC measurements and ratios were included in the IMWG diagnostic guidelines. Additionally, serum FLC determination is used commonly to monitor disease progression during treatment, although there is no consensus on how FLC determinations should best be utilized in monitoring protocols.
In addition to their role in diagnosing multiple myeloma and in monitoring disease progression and treatment, serum FLCr also provide information on prognosis for multiple myeloma patients at several points in disease survival. Evidence shows serum FLCr and absolute FLC of the involved light chain to be significantly prognostic for patient survival. These ratios have also been shown to be independent and can be combined with risk stratification from the international staging system. Other studies have found serum FLCr prognostic in the rate and degree of normalization following treatment. Normalization of serum FLCr has also been included in the definition of stringent complete response in the IMWG response criteria.
Abnormal serum FLCr along with increased clonal bone plasma marrow cells and elevated serum monoclonal protein have also been associated with increased risk of disease progression in smoldering multiple myeloma. Like smoldering myeloma, serum FLC have been incorporated into myeloma working group guidelines as a risk stratification factor. In addition, serum FLC measurements have shown utility in determining the prognosis of and in detecting and monitoring light chain amyloidosis.
Measurement of intact Ig’κ, Ig’λ, and Ig’κ/Ig’λ ratio has been made possible with the recent availability of heavy light chain immunoassays (such as The Binding Site’s Hevylite) or the intact Ig subsets: IgGκ, IgGλ and IgAκ and IgAλ, Ig’κ, Ig’λ, and Ig’κ/Ig’λ
ratios. These assays utilize epitopes which span specific intact heavy and light chain pairings.
As such they quantitatively measure concentrations of specific antibody species such as IgGκ, IgGλ, IgAκ, and IgAλ. Of note, the Hevylite assay is not specific to one monoclonal protein. However, the presence of monoclonal protein is usually indicated by an abnormal heavy light chain kappa/lambda ratio. There is substantial indication that heavy light chain Ig’κ/Ig’λ ratios may have diagnostic and monitoring value for multiple myeloma. They also may be valuable in monitoring disease progression, but there are no consensus guidelines on their use. These heavy light chain assays appear to correlate well with total immunoglobulin measurements.
In concordance with IFE, monoclonal immunoglobulins are typically quantified by SPEP and total immunoglobulin determinations, although universal correlation between these methods is not achieved. SPEP presents an analytical challenge in that the presence of overlapping non-immunoglobulin proteins makes it difficult to accurately determine monoclonal protein concentration.
Myeloma patients also experience changes in hematocrit and blood volume, resulting in variable serum concentrations of immunoglobulins independent of tumor production, as well as variable of IgG brought on by differing clearance rates at high monoclonal concentrations. Measuring heavy light chain (Ig’κ/Ig’λ) ratios may alleviate these challenges. Recent reports have also suggested that heavy light chain ratios and suppressed concentrations of non-clonal heavy light chain proteins of the same class are of apparent prognostic significance in MGUS and in multiple myeloma.
The Future
As our understanding of the utility of these markers deepens, we understand better how to use them most effectively in combination with other methods for monoclonal protein detection. As an example, the combination of SPEP and serum FLC alone has been shown sufficient to screen/detect disease in new multiple myeloma patients with 100% sensitivity. As effective test utilization initiatives progress there will be increased pressure to follow the recommended testing algorithm, although adoption of this protocol has been uneven. New technologies and guidelines such as mass spectrometric characterization of monoclonal proteins may be on the horizon. In addition, the rapid developments in myeloma disease molecular testing will likely spur continued development of more comprehensive and incisive screening and monitoring protocols.
References
- Palumbo A, Anderson K. Multiple myeloma. N Engl J Med 2011;364:1046–60.
- Ludwig H, Miguel JS, Dimopoulos MA, et al. International Myeloma Working Group recommendations for global myeloma care. Leukemia 2014;28:981–92.
- Dispenzieri A, Kyle R, Merlini G, et al. International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders. Leukemia 2009;23:215–24.
- Katzmann JA, Kyle RA, Benson J, et al. Screening panels for detection of monoclonal gammopathies. Clin Chem 2009;55:1517–22.
- van Rhee F, Bolejack V, Hollmig K, et al. High serum-free light chain levels and their rapid reduction in response to therapy define an aggressive multiple myeloma subtype with poor prognosis. Blood 2007;110:827–32.
- Boota M, Bornhorst J, Singh Z, et al. Novel prognostic modalities in multiple myeloma. In: Hajek R, ed. Multiple myeloma—A quick reflection on the fast progress. InTech Press 2014.
- Kyle RA, Durie BG, Rajkumar SV, et. al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia 2010;24:1121–7.
- Keren DF. Heavy/light-chain analysis of monoclonal gammopathies. Clin Chem 2009;55:1606–8.
- Katzmann JA, Clark R, Kyle RA, et al. Suppression of uninvolved immunoglobulins defined by heavy/light-chain pair suppression is a risk factor for progression of MGUS. Leukemia 2013;27:208–12.
- Bradwell A, Harding S, Fourrier N, et al. Prognostic utility of intact immunoglobulin Ig’kappa/Ig’lambda ratios in multiple myeloma patients. Leukemia 2013;27:202–7.
Joshua Bornhorst, PhD, DABCC, FACB, is an associate professor of pathology and director of chemistry, immunology, point-of-care testing, and pediatric laboratories at the University of Arkansas for Medical Sciences in Little Rock.
+Email: [email protected]