How old are your ovaries? While a woman’s chronological age is associated with her ovarian age, it is actually not the most accurate indicator of ovarian reserve. The term ovarian reserve refers to the number of primordial follicles—oocytes—in the ovary. Ovarian reserve status can be used as an aid in predicting the onset of menopause, for family planning, or in treating infertility. The serum marker follicle stimulating hormone (FSH) has conventionally been used to assess ovarian reserve, but like age, it has significant limitations. Anti-müllerian hormone (AMH) is emerging as the serum marker that provides the most accurate assessment of ovarian reserve and with promising emerging clinical applications.
The Biology of AMH
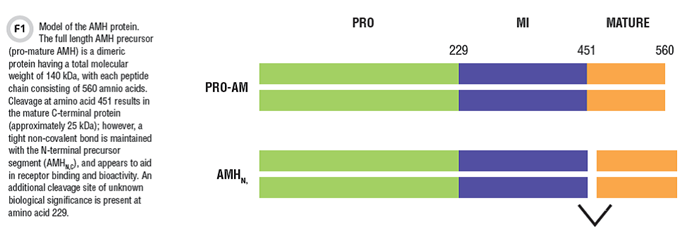
AMH is part of the TGF-beta family of growth factors, and like other members of this protein family, it is synthesized as a larger inert precursor protein (140 kDa), called pro-mature AMH. Post-translational processing of the molecule includes cleavage at amino acid 451, resulting in an N-terminal pro region and a C-terminal mature region. These peptide fragments stay in a tight non-covalent complex, and circulate as a molecule called AMHN,C (Figure 1). The AMHN,C complex enhances receptor binding and bioactivity at target tissues.
AMH was discovered and named on the basis of its role in prenatal gender differentiation. The Y chromosome directs differentiation of the gonad into testes. Sertoli cells secrete AMH, causing regression of the müllerian ducts, which would otherwise develop into the female secondary sex organs: fallopian tubes, uterus, and vagina.
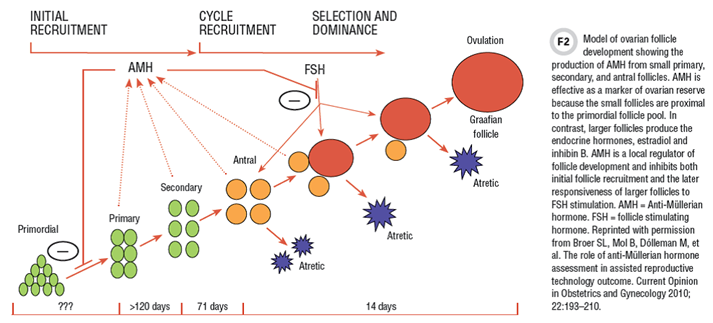
The ovaries do not secrete AMH in early prenatal development. However, by mid-gestation, ovaries’ small developing follicles begin to produce and secrete AMH. High levels of AMH mRNA and protein are observed in the granulosa cells of primary, secondary, and preantral follicles. AMH inhibits recruitment of follicles from the primordial pool, and also inhibits the response of larger follicles to pituitary gonadotropin stimulation.
AMH has the advantage over other serum hormone markers for assessment of ovarian reserve in that it is directly produced by the small follicles of the ovary, which are most proximal to the primordial pool. This contrasts with other serum markers that are either not directly secreted by the ovary or are made by more developed follicles. For example, FSH is an indirect measure of ovarian reserve being produced by the pituitary gland, not the ovary. Other candidate markers, such as inhibin B and estradiol, are produced from larger follicles after FSH stimulation, and are more distal to the primordial pool (Figure 2). Studies have shown that serum AMH has the highest correlation with ovarian primordial follicle number, among all serum hormones tested.
AMH has another important advantage over other biomarkers of ovarian reserve in that measuring it is more convenient for patients. AMH secretion is independent of gonadotropin regulation, with relatively stable serum levels throughout the menstrual cycle. As a result, samples can be collected without regard to cycle day. Again, this contrasts with other candidate markers like FSH, inhibin B, and estradiol, which all have variable patterns of secretion across the menstrual cycle. AMH has also proven to have the highest reproducibility between consecutive menstrual cycles.
Clinical Utility
The majority of data exploring the clinical utility of AMH in women comes from research in the infertile population. Most studies use a model that compares AMH levels before and after infertility treatment. Numerous publications have documented a strong relationship between pre-treatment AMH levels and ovarian response to gonadotropin stimulation. For example, investigators in our institution demonstrated that oocyte retrieval for in vitro fertilization was more successful in women with a higher AMH level than with a lower AMH level, with 18 versus four oocytes retrieved, respectively.
A summary analysis of the studies published to date suggests that AMH can predict poor ovarian response to gonadotropin stimulation with about 80% sensitivity and 80% specificity. AMH is less useful in predicting pregnancy success—not surprising given that AMH is a marker only of ovarian health.
How might AMH be used in assisted reproductive programs? Serum AMH levels can be used as an aid in counseling couples about their prospects for successful outcomes and in considering treatment options. Women with very low AMH levels are likely to have poor oocyte retrieval—critical information when considering the financial and emotional costs of treatment. If a couple opts for assisted reproduction, the AMH level can guide the specific treatment protocol. Women with relatively low serum AMH levels may be treated most aggressively, with the highest doses of gonadotropin stimulation, in order to maximize the chances of a good oocyte yield. On the other hand, those with relatively high serum AMH levels are at risk for ovarian hyperstimulation syndrome and should be treated more conservatively. Studies have shown that AMH-guided cycles result in fewer cases of ovarian hyperstimulation than do FSH-guided treatment cycles. Not only is this a safer protocol, but it is also more economical because of lower stimulation doses and fewer hospitalizations.
AMH also has a clinical role in fertile women with normal menstrual cycles. Ovarian reserve undergoes a natural attrition with age in all women, although the rate of decline varies between individuals. Menopause is essentially the complete loss of ovarian reserve. Predicting menopause onset has value for family planning and the medical care of related health issues.
As menopause approaches, serum FSH levels increase substantially, but new data show that this occurs only after a significant decline in serum AMH levels. Serum AMH levels decline in women with advancing chronological age as the primordial follicle pool is depleted. In fact, AMH levels become undetectable in serum as early as 5 years before the onset of menopause. In this way, AMH may be used as an “egg timer.” Assessing AMH levels can determine a woman’s ovarian reserve status and estimate the time remaining before reproductive senescence. Normative data for the decline in AMH levels with age, and tools for predicting menopause, are being developed.
AMH Assays
Several AMH immunoassays have been available over the past 20 years. Initial studies in the 1990s were conducted using Beckman Coulter’s Immunotech or Diagnostic Systems Laboratories methods, the latter of which has been discontinued. Beckman Coulter modified its original assay and introduced the widely used Generation II product. The antibodies used in the Gen II assay have now been adopted in the first commercial automated AMH assays from both Roche Diagnostics and Beckman Coulter. Most recently, Ansh Laboratories introduced a new family of AMH assays using novel surface epitope antibodies. In the United States, all available AMH assays are currently labeled for research use only.
Levels of AMH in serum can be very low in some instances, such as in the years leading to the onset of menopause. The existing assay methods have been unable to reliably measure serum AMH levels in such samples. Manufacturers have recognized this limitation. In response, the Ansh Labs picoAMH assay offers high sensitivity measurement, which is important for select applications.
Another limitation of current AMH assays is the lack of specificity for the mature C-terminal protein. All the available methods employ at least one antibody that recognizes an epitope on the precursor region of the AMH molecule. This means that precursor and bioactive forms of AMH in serum are not being distinguished and reported levels actually reflect a combination of forms, or “total AMH.” Whether the secreted forms of AMH might vary among healthy women or in different disease states remains to be seen. Assays with specificity for mature AMH, in addition to precursor-specific options, would be helpful for this investigation.
The variety of AMH assay options has led to some confusion among clinical laboratorians and scientific researchers. The first issue is the lack of a universal standard for calibration between methods. In addition, there have been reports of apparent AMH molecule instability under certain handling and storage conditions. Levels of AMH appeared to increase significantly with storage at room temperature or -20°C. After investigation, complement proteins were found to interfere with antigen binding to the capture antibody in the Gen II assay, leading to artificially reduced serum AMH levels. Since complement varies between samples and under different storage conditions, this factor led to high variability in AMH levels both within and between laboratories using the assay.
To deal with this problem, a new protocol was introduced in 2013 for the Gen II assay, in which all samples are mixed with buffer before adding to the AMH antibody-coated microtiter plate, thus avoiding the interference of complement proteins. Laboratorians should use caution when interpreting absolute levels of AMH, especially for data published using the Gen II assay method before introduction of the pre-mix protocol.
Collectively, raised awareness of assay limitations, attention to sample handling, improved protocols, newer methods, and the new universal standard will lead to more reproducible AMH levels and the ability to better define normative data in the near future.
Covariates
As AMH is studied in women’s reproductive health, other variables emerge that can affect serum levels. For example, several studies have now demonstrated that there is a difference in age-related AMH levels among Caucasian, African-American, Hispanic, and Chinese women. Perhaps even more intriguing, the rate of AMH decline with advancing chronological age also differs among these groups. African American women, despite having the lowest AMH levels at younger ages, demonstrate the most gradual rate of decline with age. Researchers have not yet identified the genetic and environmental influences responsible for this difference.
Most studies find that serum AMH levels are lower in women with a higher body mass index (BMI). There is an inverse correlation of BMI and serum AMH levels in women with normal menstrual cycles, as well as in women with infertility (polycystic ovary syndrome). Whether this is a simple effect of AMH dilution in the higher blood volume of larger women, or whether there is actual decreased synthesis and production, is unknown. Nevertheless, AMH data should be interpreted in the context of a woman’s BMI, as well as her age.
Smoking is known to have a negative effect on ovarian health, and serum AMH levels reflect this with lower levels observed in current smokers. The impact is transient given that prior smokers have serum AMH levels within the range of age matched non-smokers. Oral contraceptive use, of various types, has also been shown to cause a dramatic reduction in serum AMH levels in some, but not all, studies. These and other potential variables affecting serum AMH levels warrant further investigation.
Conclusions
Proper utilization of AMH provides essential reproductive health information for women. All women potentially could benefit from AMH data—from young women with irregular menstrual cycles, to fertile adults seeking to conceive, as well as infertile women, or those with cancers. AMH provides a window to the ovary, giving a view not previously available from other endocrine markers.
The immediate challenges ahead lie in standardizing assays and in developing robust and reproducible normative data against which individual patient results can be interpreted. Tools to aid in predicting fertility, preferably taking advantage of multiple marker algorithms, would also advance care.
Figure 3
Correlation analyses for the number of antral follicles and serum levels of AMH, inhibin B, estradiol, FSH and luteinizing hormone (LH) on day 3 of the menstrual cycle. Serum AMH levels had the highest correlation with antral follicle count. Reprinted with permission from Fanchin R, Schonauer LM, Righini C et al. Serum anti-mullerian hormone is more strongly related to ovarian follicular status than serum inhibin B, estradiol, FSH and LH on day 3. Human Reproduction 2003; 18: 323-7.
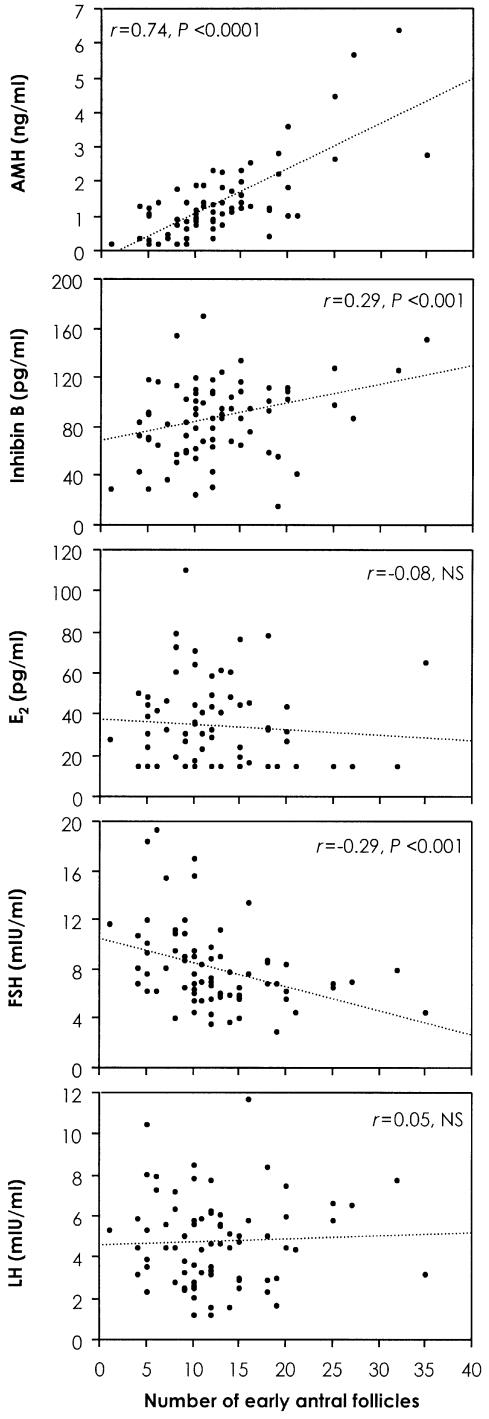
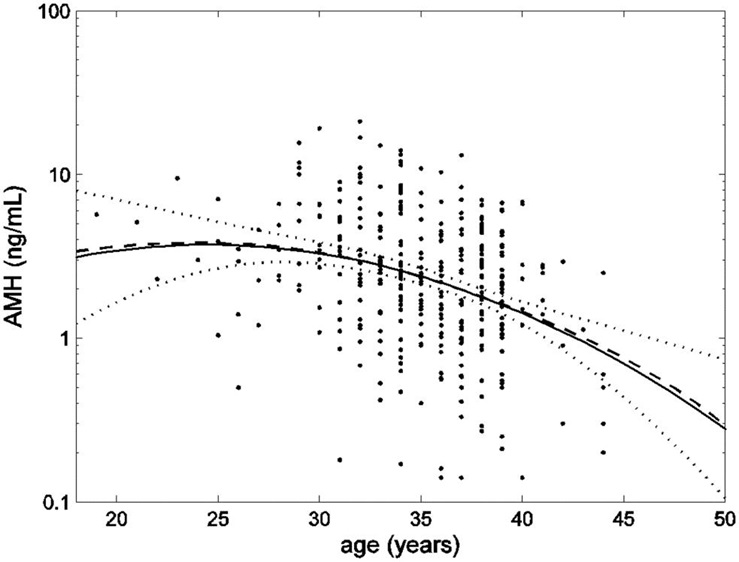
Figure 4
AMH data (dots) plotted on a logarithmic scale with smoothed mean (dashed line) and an estimate of the mean as a quadratic function of age (solid line) with upper and lower 95% confidence limits (dotted lines). Reprinted from La Marca A, Sighinolfi G, Papaleo E, Cagnacci A, Volpe A, et al. (2013) Prediction of Age at Menopause from Assessment of Ovarian Reserve May Be Improved by Using Body Mass Index and Smoking Status. PLoS ONE 8(3): e57005. doi:10.1371/journal.pone.0057005.
Suggested reading
- Broer SL, Broekmans FJM, Laven JSE, et al. Anti-Müllerian hormone: Ovarian reserve testing and its potential clinical implications. Hum Reprod Update 2014; 20:688–701.
- Dewailly D, Andersen CY, Balen A, et al. The physiology and clinical utility of anti-Müllerian hormone in women. Hum Reprod Update 2014;20:370–85.
- Han X, McShane M, Sahertian R, et al. Pre-mixing serum samples with assay buffer is a prerequisite for reproducible anti-Müllerian hormone measurement using the Beckman Coulter Gen II assay. Hum Reprod 2014;29:1042–8.
- Iliodromiti S, Kelsey TW, Wu O, et al. The predictive accuracy of anti-Müllerian hormone for live birth after assisted conception: A systematic review and meta-analysis of the literature. Hum Reprod Update 2014;20:560–70.
- Knowlton NS, Craig LB, Zavy MT, et al. Validation of the power model of ovarian nongrowing follicle depletion associated with aging in women. Fertil Steril 2014;101: 851–6.
- LaMarca A, Sunkara SK. Individualization of controlled ovarian stimulation in IVF using ovarian reserve markers: From theory to practice. Hum Reprod Update 2014;20:124–40.
- Nelson S. Biomarkers of ovarian
response. Fertil Steril 2013;99:
963–9.
- Robertson DM, Kumar A, Kalra B, et al. Detection of serum antimüllerian hormone in women approaching the menopause using a sensitive antimüllerian hormone enzyme-linked immunosorbent assays. Menopause 2014;21:
1277–86.
- Rustamov O, Smith A, Roberts SA, et al. The measurement of anti-Müllerian hormone: A critical appraisal. Clin Endocrinol Metab 2014;99:723–32.
- Visser JA, Schipper I, Laven JSE, et al. Anti-Müllerian hormone: An ovarian reserve marker in primary ovarian insufficiency. Nat Rev Endocrinol 2012;8:331–41.
Geralyn Lambert-Messerlian, PhD, FACB, is professor of pathology and laboratory medicine at the Alpert Medical School of Brown University and director of medical screening and special testing at Women and Infants Hospital in Providence, Rhode Island.
+email: [email protected]
Disclosure: The author has a financial interest in Beckman Coulter and Ansh Labs.