Chronic Kidney Disease (CKD) is a common clinical condition with significant adverse effects for patients. Since 2002, CKD has had strictly defined criteria for diagnosis and staging based almost entirely on clinical chemistry laboratory results. The 2002 guideline produced by the Kidney Disease Outcomes Quality Initiative (KDOQI) was very influential on laboratory and clinical practice, both in scope—for example with the introduction of routine reporting of the estimated Glomerular Filtration Rate (eGFR)—and in reach, affecting clinical practice throughout the world (1).
In late 2012, this guideline was superseded by the 2012 Kidney Disease, Improving Global Outcomes (KDIGO) Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease (2). A product of large scale international collaboration, the KDIGO guideline builds on many of the initiatives of the previous version and also introduces new recommendations for laboratory testing and interpretation. This guideline has already made a significant impact in the global setting. As laboratory medicine professionals, we need to understand the needs of requesting clinicians and provide results which support decisions based on evidence-based clinical guidelines. To do so, we must understand both the guideline and its optimal implementation in our local settings.
The KDIGO recommendations for laboratory practice are summarised here, along with additional information and discussion. In this article, we have used the same sections and headings as in the KDIGO guidelines to enable cross-reference with the original publication.
Changes in the New Guideline
Definitions of disease diagnosis and staging may seem tedious compared with the care of individual patients. However, correct diagnosis depends on clear, consistent, and easily applied definitions. Researchers need accurate clinical definitions to select populations for clinical trials; clinicians depend on the same definitions in routine practice to apply trial findings.
The 2002 KDOQI guidelines created a global paradigm shift in CKD assessment and management (1). This document formally entrenched the term “chronic kidney disease” in place of “chronic renal failure” to assist with patient understanding of the condition. KDOQI 2002 also clearly stated the definition and staging of CKD based on GFR, and confirmed the routine use of eGFR rather than interpreting serum creatinine alone.
Now, the KDIGO update is already further refining the understanding and management of CKD. It is important to recognize that the KDIGO guideline aims to be evidence-based and suitable for consideration in all clinical settings worldwide. The authors understood that health delivery is different in different countries and locations due to differences in resources, patterns of disease, laboratory facilities and other factors, and that implementation should be considered within the local context. Thus, the document should not be seen as a prescription for the exact way to manage CKD, but as a set of best practices that should be considered locally.
The 2012 KDIGO Guideline is divided into five chapters, of which Chapter 1, Definition and Classification of CKD, has the greatest direct relevance for laboratories with specific recommendations for estimating GFR and urine protein. Later chapters frequently refer to these and other laboratory results.
The key concepts in this guideline already have been adopted in many parts of the world, and as our clinical colleagues consider implementation, we as laboratorians need to understand the laboratory requirements specified in the document. Many key thinkers in this field are of the strong opinion that in order to achieve the best clinical outcomes, any actions by the laboratory need to have the support and active involvement of clinicians to ensure appropriate interpretation and patient management based on the results of testing. Similarly, clinical activities are dependent on high quality laboratory services to reach their optimal benefit.
Definition and Staging of CKD (Guideline sections 1.1, 1.2)
The concepts behind the definition of CKD remain unchanged, relying on a reduced GFR and/or evidence of kidney damage. The threshold for a diagnosis of CKD remains at a GFR below 60 mL/min/1.73m2 for more than 3 months, while the definition of kidney damage is clarified and expanded. Kidney damage now includes a precise definition of albuminuria, urine sediment abnormalities, electrolyte and other abnormalities due to tubular disease, abnormalities detected by histology, and structural abnormalities detected by imaging or history of kidney transplantation.
KDIGO refines CKD staging in several ways compared to the previous document. GFR remains a key determinant for 5 categories as previously defined, with the addition of a split of category 3 into 3a (GFR 45–59 mL/min/1.73m2) and 3b (GFR 30–44 mL/min/1.73m2). A major change is the formal combination of GFR categorization with a measure of albuminuria as a marker of renal damage (See Figure 1). The guideline divides albuminuria into three categories providing a two-dimensional matrix of severity. This matrix, sometimes referred to as a heat map, can be used to assign risk for CKD outcomes (Guideline section 1.3), risk of progression (2.1.4), and guide management decisions such as further monitoring and planning for renal replacement therapy (5.1.2). An additional feature of the staging process is a classification based on the cause of kidney disease where this is known. The full classification combines the cause, GFR, and albuminuria (CGA classification). For example, a patient with an eGFR of 40 mL/min/1.73m2 and albumin creatinine ratio (ACR) of 50 mg/mmol due to diabetic nephropathy would be diabetes G3bA2.
The GFR and albuminuria categories, together with the cause and other relevant factors, can be used to predict prognosis in CKD, both for outcomes—such as the need for renal replacement therapy—and for complications (Guideline section 1.3). Other factors that would be considered include age, blood pressure, known vascular disease, and other conditions. Predicting prognosis is a clinical activity and not based solely on laboratory results.
Evaluation of CKD (Guideline section 1.4)
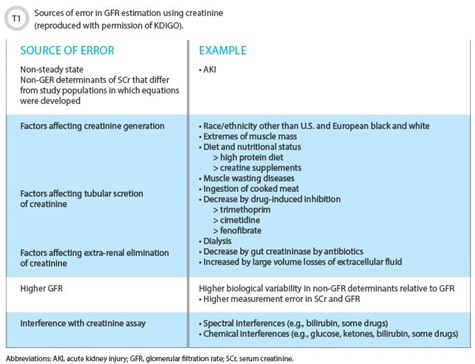
The guideline deals with laboratory issues in considerable detail. The section on evaluation of CKD carries the majority of the laboratory-specific recommendations and thus is of greatest immediate interest to laboratory scientists. Serum creatinine and an estimated eGFR remain recommended for initial assessment (1.4.3.1), with a confirmation that creatinine concentration alone not be used (1.4.3.3). The guideline also recommends that clinicians understand conditions in which eGFR may be less accurate. This is an area where laboratories should offer assistance, specifically with educational support around creatinine testing. The guideline includes an excellent summary of factors affecting creatinine-based GFR estimates (See Table 1).
The guideline also specifies the analytical and reporting recommendations for serum creatinine measurements. Serum creatinine should be measured “using a specific assay with calibration traceable to the international standard reference materials and minimal bias compared to isotope-dilution mass spectrometry (IDMS) reference methodology” (1.4.3.4). The authors expand on this statement, noting that reference materials, reference methods, and reference laboratories suitable for establishing assay traceability are listed on the database produced by the Joint Committee for Traceability in Laboratory Medicine (JCTLM; www.bipm.org/jctlm).
Laboratorians must assess the information provided by manufacturers regarding their creatinine assays, and traceability to JCTLM listed references should be considered a minimum standard. The guideline also notes a preference for enzymatic creatinine assays compared to Jaffe assays. Enzymatic assays lead to reduced between-laboratory bias, fewer samples with interferences, and lower within-laboratory imprecision. The guideline does not provide quality standards for creatinine assays in terms of allowable bias or imprecision. Laboratories may derive these themselves or adopt criteria such as those endorsed by the National Kidney Disease Education Program (3).
The document also comments on reporting serum creatinine results, recommending two decimal places when reporting in mg/dL and to the nearest whole number when reporting in µmol/L. Laboratories that need to change to IDMS-aligned assays should be aware that this may require a change in reference intervals that should be discussed with clinicians prior to implementation. While this change may be essentially complete in most developed countries, it is far from true in developing countries, but the guidelines have been written for a global audience.
The authors retained the prior recommendation to report an eGFR with requests for serum creatinine in adults. The guideline recommends using the 2009 CKD-EPI equation unless another equation has been shown to be better in the local population (1.4.3.4). This recommendation is stated in a manner which allows a rational approach to the sometimes vexing question of which eGFR formula to use. The CKD-EPI formula is strongly suggested as the formula of choice; however, if there is evidence for using an alternate formula in a particular clinical environment, then that is acceptable. An example of this would be the robust activity to develop an improved equation for use in China (4).
Staying with an alternative equation without high quality evidence is not supported. Laboratories should report eGFR in mL/min/1.73m2 to the nearest whole number and ensure that users are aware of which GFR estimating equation is used. eGFR <60 mL/min/1.73m2 should be reported as “decreased” and a flag such as an asterisk might be used for this purpose. Laboratories should also be involved in user education concerning the limitations of GFR estimating equations (1.4.3.3).
The purpose of standardising apparently trivial matters such as number of significant figures or specifying the formula used is aimed at minimising confusion for users of the laboratory. Rather than wondering why a result from one laboratory is presented differently to another, the focus remains on the result itself. The benefits of standardization are only apparent if there is widespread adoption in a city, state, country, or beyond. This obviously requires cooperation—between laboratories, as well as with nephrology and other clinical organizations.
Use of Cystatin C (1.4.3.2)
The guideline introduces a proposed routine use for serum cystatin C with a recommendation that this analyte be used to further investigate stage 3a if there are no positive markers of kidney damage (1.4.3.5). The rationale for this approach is based on findings that patients reclassified from stage G3aA1 (indicating CKD) to G2A1 (indicating no CKD) using cystatin C-based eGFR have the same prognosis as other non-CKD patients, significantly reducing the total number of CKD patients.
 Laboratories measuring serum cystatin C should use an assay with calibration traceable to the international standard reference material (ERM/DA471/IFCC); report cystatin C in mg/L to two decimal places; report eGFR from serum cystatin C in addition to the serum cystatin C concentration; use the 2012 CKD-EPI eGFRcys equation, and specify the equation used whenever reporting eGFRcys. Similar recommendations are made if using an equation with both serum creatinine and cystatin C as inputs.
Laboratories measuring serum cystatin C should use an assay with calibration traceable to the international standard reference material (ERM/DA471/IFCC); report cystatin C in mg/L to two decimal places; report eGFR from serum cystatin C in addition to the serum cystatin C concentration; use the 2012 CKD-EPI eGFRcys equation, and specify the equation used whenever reporting eGFRcys. Similar recommendations are made if using an equation with both serum creatinine and cystatin C as inputs.
Formal GFR Measurements (1.4.3.8)
Most routine clinical chemistry laboratories do not perform formal GFR measurement, however laboratorians should be aware of facilities that do. The guideline carries some important background information on the strengths and weaknesses of the gold standard GFR measurement techniques. Not all formal GFR measurements are equal, and some understanding can help interpret the scientific literature about selecting the best eGFR formula.
Evaluation of Albuminuria (1.4.4)
The guideline’s recommendation to incorporate assessment of albuminuria into the definition and staging of CKD may result in the most significant changes in routine laboratory practice. Laboratories must not only provide this testing service, but also establish appropriate interpretive limits. The preferred sample is an early morning spot sample. This testing can be performed using the following methods—from most to least preferred: laboratory measurement of an albumin-to-creatinine ratio (ACR); laboratory testing of a protein-to-creatinine ratio; reagent strip urinalysis with automated reading; or reagent strip urinalysis with manual reading.
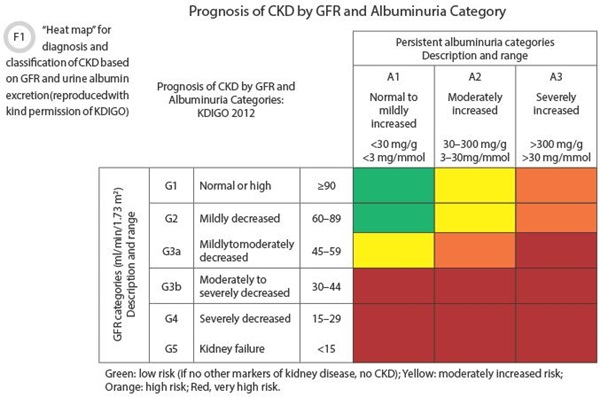
The authors based their recommendation for albumin in preference to protein on greater sensitivity for small increases in protein loss and the opportunity to standardize assays. The use of the ratio to creatinine reduces within-subject variability by minimizing the effect of changes in hydration and urine concentration. Decision points are provided in Figure 1 for interpreting the ACR. As with eGFR, there are factors related to collection and interpretation in which laboratories have a role to play in terms of educating clinicians. A table of factors affecting ACR results is reproduced in Table 2.
Conclusions
The 2012 Clinical Practice Guideline was developed to support high quality, evidence-based practice for identifying and managing CKD. Implementation of this guideline requires good laboratory practice. Some of the components described may not be appropriate in all settings. For example, use of cystatin C may be considered too expensive in some healthcare settings. By contrast, providing albuminuria testing is now a fundamental requirement for use of the latest classification scheme.
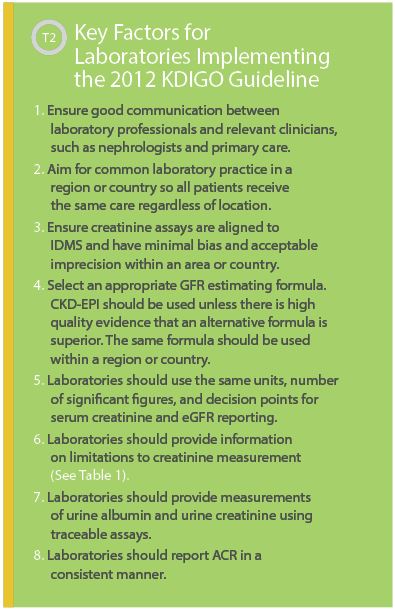
We live in an environment in which the practice of medicine is a team activity. Physicians cannot diagnose and treat CKD without proper laboratory services, and laboratory results are irrelevant if they are not requested and interpreted correctly. The basis of this is close cooperation between clinical and laboratory colleagues, and the 2012 KDIGO guideline provides an excellent, evidence-based resource upon which to build a high quality testing service. A brief list of key factors for implementing the guideline is provided in Table 2.
References
- KDOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. https://www.kidney.org/professionals/kdoqi/guidelines_ckd/toc.htm (Accessed March 2014).
- Kdigo 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013;3:1–150.
- Myers gl, Miller WG, Coresh J, et al. for the National Kidney Disease Education Program. Laboratory working group recommendations for improving serum creatinine measurement: A report from the laboratory working group of the National Kidney Disease Education Program. Clin Chem 2006;52:5–18.
- Zhu Y, Ye X, Zhu B, et al. Comparisons between the 2012 new ckd-epi (Chronic Kidney Disease Epidemiology Collaboration) equations and other four approved equations. Plos one 2014;9:e84688.
Graham Jones, MBBS, BSc(med), DPhil, FRCPA, FAACB, is a chemical pathologist at St. Vincent’s Hospital, Sydney, Australia, and a conjoint associate professor at the University of New South Wales, Australia. He has been active in the field of laboratory testing for chronic kidney disease since 2004 as co-chair of the Australasian eGFR working group, and more recently as co-chair of the IFCC/WASPaLM Task Force on Chronic Kidney Disease.
+Email: [email protected]
