Many clinical laboratories are breaking from the current syphilis screening algorithm recommended by the Centers for Disease Control and Prevention (CDC) in order to use more specific, automated assays. However, many providers are still confused about how to interpret test results and what follow-up testing, if any, is required. This article reviews current syphilis assays and, using four case studies, explains how laboratories can implement the new algorithm and advise clinicians.
Fundamentals
Syphilis is a chronic, sexually transmitted disease caused by the spirochete, Treponema pallidum. The clinical manifestations of syphilis are often non-specific and may be progressive if the disease is not diagnosed and treated appropriately. Although laboratory methods such as dark field microscopy and real-time PCR have been used in the past, serology represents the most common diagnostic approach in cases of syphilis.
Serologic tests fall into two categories based on the type of antibodies detected. Non-treponemal assays, such as rapid plasma reagin (RPR) and Venereal Disease Research Laboratory (VDRL) assays, detect the reactivity of antibodies in serum or cerebrospinal fluid (CSF) against cardiolipin-cholesterol-lecithin antigens, which, though not specific to T. pallidum, are present in most patients with syphilis. In contrast, treponemal assays detect specific IgG- and/or IgM-class antibodies directed against T. pallidum, and include fluorescent treponemal antibody absorption (FTA-ABS), Treponema pallidum particle agglutination (TP-PA), enzyme immunoassay (EIA), and multiplex flow immunoassay (MFI).
Traditionally, laboratories have screened serum samples using a non-treponemal test and only used a treponemal assay to confirm screen-positive samples (Figure 1). This algorithm is currently recommended by CDC (1) due in part to the fact that non-treponemal tests are inexpensive (2), relatively easy to perform, show good correlation with disease status (3, 4), and may be used to follow a patient’s response to therapy. Despite these advantages, non-treponemal tests are subjective and non-specific, and may result in false-positive results, especially in areas with a low prevalence for the disease. Furthermore, tests such as RPR require manual processing—a significant limitation for clinical laboratories testing a high volume of samples.
Now, growing numbers of clinical laboratories have adopted a reverse sequence screening algorithm that begins with a treponemal assay, such as EIA or MFI, and tests screen-positive samples by RPR. Under a reverse sequence screening algorithm, samples that are positive by the screening test but negative by RPR should then be tested by a second treponemal assay for confirmation (Figure 2) (1).
Case Studies
Reverse screening offers a number of advantages, including automated screening on a high-throughput testing platform, and an objective and specific screening assay. Furthermore, recent studies have demonstrated that reverse sequence screening may detect more cases of early or latent syphilis compared to the traditional screening algorithm (5). However, reverse screening has generated a significant amount of confusion, particularly when treponemal assay and RPR results are discordant. In this situation, providers are often left to ask, “Does my patient have syphilis?” and “Given these results, what do I do next?”
The following four cases deal with these questions, covering different syphilis serology result profiles. Each case includes recommended laboratory testing, appropriate clinical interpretation, and follow-up.
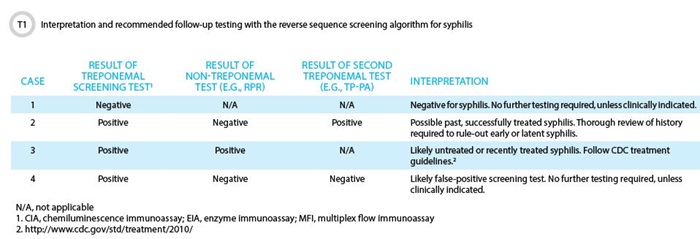
Case 1 > A 32-year-old female presented to her primary care physician with a painless genital lesion that had developed 2 days prior. Upon questioning, the patient indicated that she recently started a new relationship. Aside from the lesion, the patient was asymptomatic and did not have any constitutional symptoms. The provider, suspicious for syphilis, ordered syphilis testing using the reverse screening algorithm. The following day, the result returns as negative for T. pallidum IgG and no further testing was performed.
Questions to consider
- Does this result indicate that this patient does not have a syphilis infection?
- What other testing should be performed, if any?
Answer
Based on the clinical presentation and duration of the lesion, acute syphilis cannot be excluded in this patient. Additional testing for this patient might include dark field microscopy to detect spirochetes in scrapings from the lesion. Dark field microscopy, however, is not routinely available in most clinical and reference laboratories. The recommended alternative approach is to repeat serologic testing in 2–4 weeks to detect T. pallidum IgG seroconversion. Both treponemal and non-treponemal assays have limited sensitivity during the acute stages of infection.
Case 2 > A 45-year-old healthy male undergoes a complete sexually transmitted disease (STD) screen at his local clinic. All of his results are negative except for the syphilis screening assay, syphilis IgG EIA. Subsequent testing by RPR is negative, but the TP-PA assay is also positive. Confused by the results, the patient makes an appointment with a STD counselor to review the report.
Questions to consider
- Does the patient have syphilis?
- If you were the counselor, what questions would you ask the patient to better interpret the results?
Answer
These results indicate that the patient has antibodies specific to T. pallidum and that he has been exposed to the spirochete at some time in the past. The absence of a RPR titer indicates he likely does not have an active infection. Without further clinical information, these results may indicate either a previously treated syphilis or latent syphilis infection. During the return visit, the counselor should attempt to discriminate between these two scenarios by asking the patient whether he has been diagnosed and treated for syphilis in the past or if this is a new finding. If the patient does not recall being previously diagnosed with syphilis, treatment for latent syphilis should be considered.
Case 3 > A 25-year-old, HIV-positive male presented to a local clinic with a low-grade fever and a 4-day history of a nonpruritic, maculopapular rash, and generalized lymphadenopathy. The rash was symmetric in presentation, on both his trunk and extremities, including the palms and soles. In a syphilis workup, both the initial treponemal screen, syphilis IgG EIA, and the RPR were positive, with a reciprocal endpoint RPR titer of 256. The patient was diagnosed with secondary syphilis and appropriate treatment was initiated.
Questions to consider
- Why was a second, confirmatory treponemal assay such as TP-PA not performed in this case?
- Why was an endpoint RPR titer established, and how is it used?
Answer
A confirmatory treponemal assay is only necessary when results from the initial screening treponemal assay are positive and the RPR is negative. In such cases, the positive screen may be the result of a false-positive reaction, or alternatively, the RPR may be negative due to a previously resolved infection or latent disease. Differentiation between these two scenarios is possible by a second treponemal assay; if negative, the initial reactive screen can be considered falsely reactive. However, if the second treponemal assay were positive, further clinical evaluation would be necessary to distinguish between a successfully treated infection and latent disease.
The RPR titer is used by healthcare providers to monitor a patient’s response to therapy. In general, following successful treatment, the RPR should revert to negative after 1 year in patients with primary syphilis and after 2 years in patients with secondary syphilis. The presence of RPR titers following these time points has been associated with persistent infection, reinfection, or biological false-positive reactions.
Case 4 > A 32-year-old pregnant female, in her first trimester, presented to her obstetrician for her first prenatal checkup. In addition to screening for HIV, hepatitis B, and rubella, the patient was also screened for syphilis using the reverse algorithm. All tests are reported as negative except for her syphilis IgG screen. Subsequent testing by RPR and a second treponemal assay (TP-PA) were also negative. The patient remains concerned, and her provider calls the laboratory.
Questions to consider
- How would you respond to the provider?
- The provider notes that this entire scenario could have been avoided if the RPR had been performed first and recommends that the laboratory revert to the traditional syphilis screening algorithm. How would you respond?
Answer
The negative RPR and second treponemal assay suggest that the initial screen was falsely reactive. This scenario can occur in pregnant women, most often because of cross-reacting alloantibodies. Unless there is concern for recent infection, there is no indication to perform further testing. As this case shows, the reverse algorithm can be difficult to interpret, particularly in screen positive/RPR negative cases, and may lead to increased patient anxiety.
Future Directions
Due to the limitations of both the traditional and reverse sequence screening algorithms, a significant amount of effort has been made in recent years to develop new and improved methods for diagnosing syphilis. An area of interest has been to refine further the serologic testing algorithm, with the European Centre for Disease Prevention and Control (ECDC) recently recommending that a treponemal immunoassay be used to screen samples, with sera testing reactive by the initial test being tested by a second, different treponemal assay for confirmation. Results from a recent large study suggest that the ECDC algorithm may optimize sensitivity and specificity, and negate the requirement to perform a nontreponemal test for primary diagnostic purposes (6).
Another area of focus has been developing rapid and sensitive molecular assays. Unfortunately, whereas molecular tests have revolutionized the diagnosis of other infectious diseases, this technology has not proven to enhance significantly our ability to diagnose syphilis. In published studies, the sensitivity of PCR during early disease ranges from 78.4–89.1%, while the specificity is generally >95% (7, 8). During secondary syphilis, the sensitivity of PCR may be as low as 50% (8). Taken together, these reports suggest that molecular detection of T. pallidum may be useful during early disease before patients seroconvert, but likely is of limited value in later stages of disease.
In the opinion of the authors, future work should be devoted to developing sensitive, point-of-care (POC) diagnostic tests to enhance detection of early/primary syphilis. Several studies have assessed the performance of POC syphilis tests that allow for the simultaneous detection and differentiation of treponemal and nontreponemal antibodies (9, 10). We need to continue developing rapid, accurate POC tests to identify patients with early, active disease—when transmission rates are highest—so clinicians can begin treatment as early as possible.
References
- Centers for Disease Control and Prevention. Discordant results from reverse sequence syphilis screening—Five laboratories, United States, 2006–2010. MMWR Morb Mortal Wkly Rep 2011;60:133–7.
- Binnicker MJ, Jespersen DJ, Rollins LO. Treponema-specific tests for serodiagnosis of syphilis: Comparative evaluation of seven assays. J Clin Microbiol 2011;49:1313–7.
- Romanowski B, Sutherland R, Fick GH, et al. Serologic response to treatment of infectious syphilis. Ann Intern Med 1991;114:1005–9.
- Samoff E, Koumans EH, Gibson JJ, et al. Pre-treatment syphilis titers: Distribution and evaluation of their use to distinguish early from late latent syphilis and to prioritize contact investigations. Sex Transm Dis 2009;36:789–93.
- Mishra S, Boily MC, Ng V, et al. The laboratory impact of changing syphilis screening from the rapid-plasma reagin to a treponemal enzyme immunoassay: A case-study from the greater Toronto area. Sex Transm Dis 2011;38:190–6.
- Tong ML, Lin LR, Liu LL, et al. Analysis of 3 algorithms for syphilis serodiagnosis and implications for clinical management. Clin Infect Dis 2014;58:1116–24.
- Gayet-Ageron A, Lautenschlager S, Ninet B, et al. Sensitivity, specificity and likelihood ratios of PCR in the diagnosis of syphilis: A systematic review and meta-analysis. Sex Transm Infect 2013;89:251–6.
- Shields M, Guy RJ, Jeoffreys NJ, et al. A longitudinal evaluation of Treponema pallidum PCR testing in early syphilis. BMC Infect Dis 2012;12:353.
- Castro AR, Mody HC, Parab SY, et al. An immunofiltration device for the simultaneous detection of non-treponemal and treponemal antibodies in patients with syphilis. Sex Transm Infect 2010;86:532–6.
- Yin YP, Chen XS, Wei WH, et al. A dual point-of-care test shows good performance in simultaneously detecting nontreponemal and treponemal antibodies in patients with syphilis: A multisite evaluation study in China. Clin Infect Dis 2013;56:659–65.
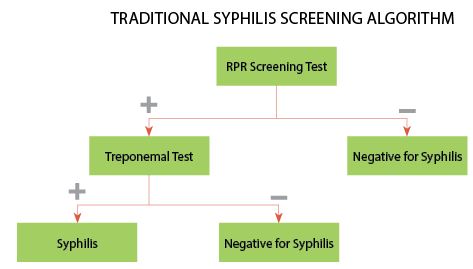
F1 In the traditional algorithm, samples are screened by a non-treponemal test, such as rapid plasma reagin (RPR). Samples testing negative by RPR do not require further analysis, and the patient is considered negative for syphilis, unless early disease is suspected. Samples testing positive by RPR should be tested by a treponemal test, such as fluorescent treponemal antibody absorption. Patients testing negative by the confirmatory test are considered negative for syphilis. Patients testing positive by RPR and the treponemal test are considered positive for untreated or recently treated syphilis.
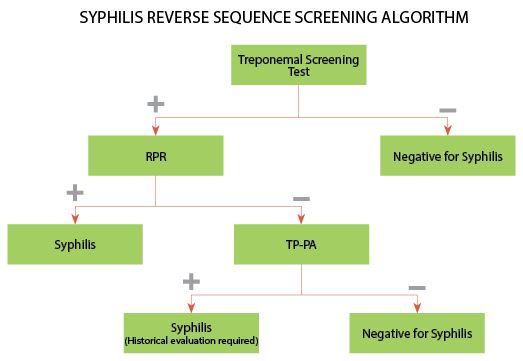
F2 In the reverse sequence screening algorithm, samples are screened by an automated treponemal test, such as a multiplex flow immunoassay (MFI). Samples testing negative by
the screening assay do not require further testing and the patient is considered negative for syphilis. Samples testing positive by the screening test are reflexed to a nontreponemal test, such as RPR. If the RPR is reactive, the patient is considered positive for untreated or recently treated syphilis.
If the RPR is nonreactive, the sample is reflexed to a second treponemal test, Treponema pallidum particle agglutination (TP-PA). If the TP-PA is negative, the screening result is typically interpreted as falsely positive. A positive TP-PA is suggestive of past, successfully treated syphilis, late/latent syphilis, or early syphilis. These results require a thorough clinical and historical evaluation to assist in the interpretation of results.

Elitza S. Theel, PhD, is an assistant professor of laboratory medicine and pathology and director of the Infectious Diseases Serology Laboratory at Mayo Clinic in Rochester, Minnesota. She is certified by the American Board of Medical Microbiology.
+Email:
[email protected]

Matthew J. Binnicker, PhD, is an associate professor of laboratory medicine and pathology and director of clinical virology at Mayo Clinic in Rochester, Minnesota. Previously, he served as director of the Infectious Diseases Serology Laboratory at Mayo Clinic and is certified by the American Board of Medical Microbiology.
+Email:
[email protected]
