Federal Panel Recommends Screening High-Risk People for Hepatitis B
The U.S. Preventive Services Task Force (USPSTF) published its final recommendation statement on screening for hepatitis B virus (HBV) infection in individuals at high risk. USPSTF gave a grade B recommendation for screening people who have the following risk factors for HBV infection: people born in countries and regions with a high prevalence of HBV infection, such as Africa, Southeast Asia, Pacific Islands, China, Middle East, Eastern Europe, and the northern countries in South America; U.S.-born individuals not vaccinated as infants whose parents were born in countries or regions with a high prevalence of HBV infection; HIV-positive individuals, injection drug users, men who have sex with men, and those living with or having sex with someone with HBV infection; and patients with weakened immune systems or undergoing treatment for kidney failure.
According to the Centers for Disease Control and Prevention (CDC), there are still as many as 2.2 million people in the U.S. chronically infected with HBV, and 15–25% of them die from cirrhosis or liver cancer. “Some people still remain at risk for getting hepatitis B and suffering from health complications of the disease, such as liver damage. It is important that these high-risk individuals be screened,” said USPSTF member Mark Ebell, MD, in a statement.
Currently, CDC recommends screening for hepatitis B surface antigen (HBsAg) with tests approved by the Food and Drug Administration, followed by a licensed, neutralizing confirmatory test for initially reactive results. A positive HBsAg result indicates acute or chronic infection.
USPSTF also noted that testing for antibodies to HBsAg (anti-HBs) and hepatitis B core antigen (anti-HBc) can make up a screening panel to help distinguish between infection and immunity. Acute HBV infection—acquired within 6 months after infection—is characterized by the appearance of HBsAg and followed by the appearance of IgM anti-HBc. The disappearance of HBsAg and the presence of anti-HBs and anti-HBc indicate the resolution of HBV infection and natural immunity. Anti-HBc persist for life, but are present only after HBV infection and do not develop in those whose immunity to HBV is due to vaccination.
USPSTF previously issued a recommendation that all pregnant women in the United States should be screened for hepatitis B, complementing this recommendation. USPSTF published the final recommendation statement online in Annals of Internal Medicine (May 27, 2014; doi:10.7326/M14-1018), as well as on the USPSTF website, www.uspreventiveservicestaskforce.org.
Medicare Will Pay for HCV Screening
Following a grade B recommendation from the U.S. Preventive Services Task Force (USPSTF), the Centers for Medicare and Medicaid Services (CMS) issued a decision memo outlining how Medicare will cover screening for hepatitis C virus (HCV).
CMS will cover screening for HCV with Food and Drug Administration (FDA) approved or cleared tests, used consistently with FDA labeling, when ordered by the beneficiary's primary care physician or practitioner within the context of a primary care setting, and performed by an eligible Medicare provider for these services.
CMS will cover HCV screening under two general conditions. First, in adults at high risk for HCV, defined as individuals with a current or past history of illicit injection drug use and those who have a history of receiving a blood transfusion prior to 1992. Repeat screening for high risk individuals is covered annually only for those who have had continued illicit injection drug use since the prior negative screening test. CMS also generally covers screening for adults who do not meet high risk criteria, but who were born from 1945 through 1965.
CDC: Measles Cases in the United States Reach 20-year High
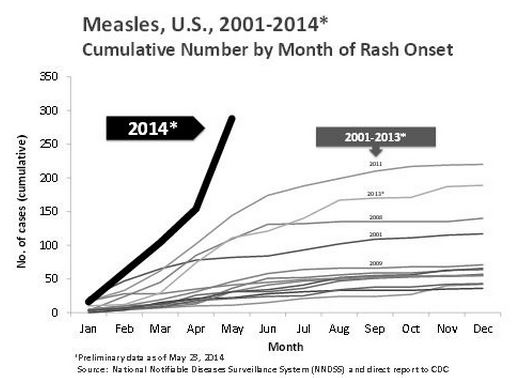
According to the Centers for Disease Control and Prevention (CDC), 288 cases of measles have been reported to the agency between January 1 and May 23, 2014. This is the largest number of measles cases in the U.S. reported in the first 5 months of a year since 1994. Nearly all of the measles cases this year have been associated with international travel by unvaccinated individuals.
“The current increase in measles cases is being driven by unvaccinated people, primarily U.S. residents, who got measles in other countries, brought the virus back to the U.S, and spread it to others in communities where many people are not vaccinated,” said Anne Schuchat, MD, assistant surgeon general and director of CDC’s National Center for Immunizations and Respiratory Diseases. “Many of the clusters in the U.S. began following travel to the Philippines where a large outbreak has been occurring since October 2013.”
Of the 288 cases, 97% were associated with importations from at least 18 countries. More than one in seven cases has led to hospitalization. Ninety percent of all measles cases in the United States were in people who were not vaccinated or whose vaccination status was unknown.
The large number of measles cases this year underscores the importance of vaccination, according to Schuchat. “Many U.S. healthcare providers have never seen or treated a patient with measles because of the nation’s robust vaccination efforts and our rapid response to outbreaks,” she said.
Patients who present with fever and rash along with cough, runny nose, or pink eye should be evaluated for measles, especially if they are unvaccinated and recently traveled internationally or were exposed to someone else who has measles or recently traveled. If healthcare providers suspect a patient with measles, they should immediately isolate the patient to help prevent the disease from spreading, immediately report the case to their local health department, and collect specimens for serology and viral testing.
Measles was declared eliminated from the United States in 2000, meaning that there was no longer continuous measles transmission for more than 12 months. The CDC report is available from www.cdc.gov/mmwr.
HHS Unveils Second Round of State Innovation Grants
Health and Human Services (HHS) Secretary Kathleen Sebelius announced new delivery system reform efforts under the Affordable Care Act that offer states tools and flexibility to improve healthcare delivery.
HHS announced 12 prospective recipients being awarded as much as $110 million in combined funding, ranging from an expected $2 million to $18 million over a 3-year period, under the Health Innovation Awards program. The program tests innovative models designed to deliver better outcomes and lower costs. Examples include projects to improve coordination between specialists and primary care physicians and to improve cardiac care. Under a second, related program called the State Innovation Model, HHS made another $730 million available.
As part of the State Innovation Model initiative, states, territories, and the District of Columbia can apply for either a Model Test award to assist in implementation or a Model Design award to develop or enhance a comprehensive State Health Care Innovation Plan. Up to 12 states will be chosen for state-sponsored Model Testing awards and up to 15 states will be chosen for state-sponsored Model Design work.
Examples of ongoing state-led healthcare innovations include advanced primary care networks supported by statewide health information technology systems. Currently, 25 states are participating in the State Innovation Models initiative. For more information on the initiative, please visit: innovation.cms.gov.