
"Doping" is as old as sporting events themselves and can be defined as the attempted use of a prohibited substance or prohibited method with the intent of improving athletic performance. As early as 800 B.C., ancient Greek athletes and Roman gladiators ingested a combination of herbs, plants, and mushrooms to gain a competitive edge and mask pain (1). With the debut of the modern Olympic Games in 1896, the practice of doping rapidly spread and different classes of compounds were used depending on the sporting event. For instance, athletes took stimulants (cocaine and amphetamines) to improve performance in speed and endurance sports, whereas they used anabolic steroids to promote muscle mass in sports requiring strength and power.
Drug testing was introduced at the Olympic Games in 1968, but had little impact on the practice of doping since the testing methods had poor analytical sensitivity that significantly reduced the window of detection. Another problem was that some compounds—notably anabolic steroids, erythropoietin and growth hormone—were used before methods to test them became available. Doping scandals continued to mount in the 1980s and 1990s and the public became acutely aware of the problem in sports. Consequently, the International Olympic Committee convened the World Conference on Doping in Sports to address this problem, and in 1999, it created the World Anti-Doping Agency (WADA) to combat sports doping and harmonize all anti-doping principles.
The goal of this article is to provide an inside view of how WADA-accredited laboratories identify athletes that use prohibited substances.
|
Table 1
WADA 2013 List of Prohibited Substances
|
|
Substances Prohibited at All Times
|
|
Anabolic Androgenic Steroids
|
| Other Anabolic Agents |
|
Peptide Hormones, Growth Factors, and Related Substances
- Erythropoiesis-stimulating agents
- Chorionic gonadotropin
- Luteinizing hormone
- Corticotropins
- Growth hormone
- Insulin-like growth factor-1
- Fibroblast growth factors
- Hepatocyte growth factor
- Mechano growth factors
- Platelet-derived growth factor
- Vascular-endothelial growth factor
|
| Beta-2 Agonists |
|
Hormone and Metabolic Modulators
- Aromatase inhibitors
- Selective estrogen receptor modulators
- Other anti-estrogenic substances
- Agents modifying myostatin function
- Metabolic modulators: insulins, peroxisome proliferator activated receptor δ agonists, PPARδ-AMP-activated protein kinase axis agonists
|
| Diuretics and Other Masking Agents |
|
Substances Prohibited In-Competition
|
| Stimulants |
| Narcotics |
| Cannabinoids |
| Glucocorticosteroids |
| Alcohol (some sports) |
| Beta Blockers (some sports, also prohibited out-of-competition in archery and shooting |
WADA and the Prohibited List
WADA is independent of governments and sports federations, but is equally represented by all stakeholders. A major element of the WADA Anti-Doping Program is the Code, a universal document dealing with all aspects of sports doping (2). WADA signatories such as national anti-doping organizations and international federations are responsible for developing a sports drug testing program in accordance with the Code and must have drug testing performed by WADA-accredited laboratories. Accredited laboratories must develop methods to detect substances on the prohibited list of substances as mandated by the World Anti-Doping Program's International Standard for Laboratories (3). At the time of this writing there were 32 accredited laboratories worldwide, with two in the United States (4).
A violation of any of the WADA anti-doping rules is considered a doping offense. Besides the presence of a prohibited substance or metabolite/marker in an athlete's sample, other activities can constitute an anti-doping rule violation. These include attempting to use a prohibited substance or prohibited method, refusing to submit a sample, failing to provide whereabouts information (location and availability for sample collection), failing to provide a sample, tampering or attempting to tamper with the doping control process, and possessing, trafficking, or attempting to traffic prohibited substances or prohibited methods. Although testing laboratories exist primarily to detect prohibited substances in athlete samples, they occasionally are asked to identify the contents in vials, pills, and powders confiscated from athletes suspected of possessing prohibited substances.
The WADA prohibited list of substances is updated annually and new substances are typically added to the list every year (5). Prohibited substances are typically grouped based on the pharmacological effect on the body (Table 1). Each class contains numerous substances; there are currently 46 exogenous steroids and 64 stimulants on the list. In the case of exogenous steroids, substances with a similar chemical structure, such as dimethazine—2 molecules of methasterone linked by an azine group—are also prohibited. Some classes of compounds are prohibited at all times whereas others are prohibited only when the athlete is in competition (Table 1). Alcohol and beta blockers are prohibited in certain sports during competition, but a few sports prohibit beta blocker use all the time. Prohibited methods are banned at all times and include manipulation of blood (transfusions, artificially enhancing oxygen delivery) and gene doping.
Figure 1
Representative urine collection kits
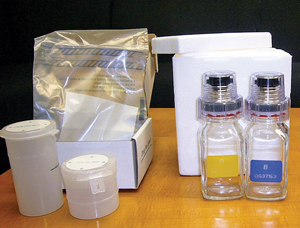 The taller plastic bottle (collection kit on the left)
is the 'A' bottle.
The taller plastic bottle (collection kit on the left)
is the 'A' bottle.
The cap and sides of the plastic
urine bottles are
covered with
tamper-evident tape
after the
lid
is sealed. The red ring
below the
cap
on
the neck of the glass bottles (collection kit on the right)
is
removed and the cap is twisted onto the bottle after filling.
The white teeth prevent the bottle from
being opened
without
breaking the plastic sleeve surrounding the cap.
Collection and Processing of Samples
Collectors witness urine collection to help prevent samples from being adulterated or substituted with fake or drug-free urine. Both the collector and athlete certify the urine sample after it is placed into two separate containers ('A' and 'B' bottle) . Urine collection kits contain either plastic or glass bottles (Figure 1). Tamper-evident tape is applied to plastic bottles to spot tampering. Glass bottles, permanently sealed after filling, can only be opened by crushing the plastic sleeve surrounding the cap with a specially designed opening device. Both bottle types are placed into a box provided with the kit and are shipped to WADA-accredited laboratories, usually at ambient temperature.
Chain of custody documentation begins when urine samples arrive at the laboratory. This includes examining each 'A' and 'B' bottle for evidence of tampering, leakage, and color/clarity, along with measuring the pH and specific gravity of the 'A' bottle. Our laboratory routinely screens extremely dilute urine samples with a specific gravity <1.006 for diuretics even when testing for diuretics is not requested. Aliquots from the 'A' bottle are then prepared for testing depending on the specific test menu. Between 5 and 7 aliquots of urine are routinely prepared for testing, with each aliquot containing from 1 to 20 mL of urine.
Sample Pretreatment
The amount of sample pretreatment needed varies depending on the screening method. Some urine testing methods such as hormone immunoassays for human chorionic gonadotropin and luteinizing hormone do not require pretreatment prior to testing. Sample pretreatment removes unwanted interfering substances and isolates specific compounds of interest. For instance, anabolic steroids first require an enzymatic hydrolysis step to deconjugate glucuronide moieties from the steroid molecules, followed by solid phase extraction to recover the relatively nonpolar steroids. We then add trimethylsilyl groups to steroid functional groups to improve chromatographic and mass spectral properties when these samples are analyzed by gas chromatography mass spectrometry (GC-MS). The complete steroid pretreatment procedure takes approximately 6 hours.
Testing Methods
We use GC or liquid chromatography (LC) separation coupled with MS detection to identify the majority of substances on the WADA prohibited list. GC-MS is routinely used to detect anabolic steroids and stimulants. However, WADA's recent lowering of the detection limit for anabolic steroid from 5 to 2.5 ng/mL (6) has forced accredited laboratories to switch to GC-triple quadrupole (GC-MS/MS) systems to meet the new requirement. We use LC-MS/MS systems to detect some anabolic steroids and most of the other classes of compounds. Exceptions include isoelectric focusing and polyacrylamide gel electrophoresis, which we use to detect recombinant erythropoietins and analogues such as peginesatide, and isotope ratio MS, which we perform to detect exogenous testosterone and testosterone precursors.
Data Analysis
GC-MS detects target compounds by comparing the retention time and relative intensities of ion fragments in unknown samples to those obtained for reference compounds. The collected data are reduced to dedicated windows consisting of selected time slices and mass-to-charge (m/z) ions corresponding to the expected retention times and mass spectral fragments for each target compound. Interfering peaks and background noise can complicate GC-MS data reading because screening methods are designed to detect entire classes of compounds and are not optimized for individual compounds. Furthermore, a single ion fragment may not be unique and might be shared by compounds. These issues limit the use of automated software programs for GC-MS data reading and require that experienced data readers carefully evaluate all data. In contrast, LC-MS/MS and GC-MS/MS measure the relative abundance of precursor/product ion pairs (transitions). Because the likelihood of a target compound and an interfering substance having the same precursor/product ion pairs is relatively small, the data usually are easier to interpret compared to GC-MS data and can be more easily evaluated by computer software.
Figure 2
GC-MS screening data for selected stimulants
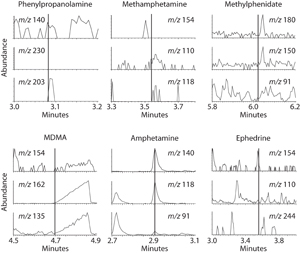 Each window consists of selected time slices and mass-to-charge
Each window consists of selected time slices and mass-to-charge
(m/z) ions corresponding to each of the target stimulants. The x-axis
is retention time and the y-axis is ion abundance. The vertical line
in each window represents the software-predicted retention time
based on reference standards analyzed in the same batch of unknown
urine samples. Note that the urine sample screens positive for amphetamine.
GC-MS screening data for six stimulants are shown in Figure 2. The retention times of reference compounds are indicated in each window by a vertical line and the relative abundance of each ion is displayed on the y-axis. Although some of the windows have considerable background signals, the urine sample appears negative for all of the stimulants except amphetamine. A peak appears at the appropriate retention time for all three m/z ions in the amphetamine windows, so this sample would be considered screen positive and would undergo confirmation testing.
Figure 3
GC-MS screening data for selected stimulants
GC-MS confirmation data for the trifluoroacetyl derivative of amphetamine
in a negative urine (A), a positive urine control (B) and the urine that screened
positive in figure 2 (C). Retention times (x-axis) and ion abundance (y-axis)
for selected m/z ions are shown in the left panels. Full-scan mass spectra
normalized to m/z ion 140 with the relative abundance of m/z 91 and m/z 118
ions are shown in the right panels. Note that all ions with a relative abundance
>10% in the control urine are also present in the screen positive urine. This data
confirms the presence of amphetamine.
For confirmation testing an additional aliquot of urine from the 'A' bottle would be analyzed separately using a testing method optimized to detect amphetamine. Ion chromatograms and mass spectra of the trifluoroacetyl derivative of amphetamine are shown in Figure 3. The negative urine (panel A) does not contain ions at the retention time of the positive urine (panel B). In contrast, the screen positive sample (panel C) contains a peak for each of the m/z ions at the same retention time as the positive control in panel B. Relative abundance of diagnostic ions in the screen positive sample (m/z 118 and 91 as a percentage of m/z 140) match those obtained for the positive urine sample and all diagnostic ions with a relative abundance of >10% that are present in the positive control are also present in the screen positive sample based on full scan mass spectra data (right panels), confirming the presence of amphetamine. The confirmation data would be reviewed by two certifying scientists before being reported as an adverse analytical finding for amphetamine and must also fulfill all identification criteria in the WADA technical document (7). If the client requests 'B' bottle testing for the substance identified in the 'A' bottle, the athlete and/or his or her representative has the right to observe the entire testing process.
Documentation and the Legal Process
WADA-accredited labs prepare documentation packages in support of adverse analytical findings that must contain a table of contents, shipping/receipt documents, and chain of custody documents for bottles and aliquoted samples, as described in the technical document (8). Other documents include a list of staff involved in the testing process, a description of the testing procedure(s), negative and positive control data, instrument performance data (chromatographic performance, tune reports, etc.), compound identification data, and an uncertainty estimate for threshold compounds. These documents must be provided for both the initial screen and the confirmation testing.
The presence or use of a prohibited substance(s) is considered an anti-doping rule violation. The burden of proof under the Code is to the comfortable satisfaction of the hearing panel. This is more than the mere balance of probability but less than proof beyond a reasonable doubt. The length of the sanction depends on the sporting organization; WADA signatories must follow Code guidelines which mandate a maximum of 2 years for the first violation. However, non-signatories like professional sports can enforce shorter periods of ineligibility.
Athletes have the right to appeal a sanction, and as part of the appeals process accredited laboratories reporting the adverse analytical finding may be called upon to testify. Depending on the case, the laboratory may be required to provide a wide variety of documents during the discovery process. Expert testimony usually involves explaining and defending data in the documentation package, providing information as to what the drug is and why it is performance-enhancing, describing side effects, as well as when and how much the athlete took. Some of these questions can be difficult if not impossible to answer since pharmacokinetic data for many of the drugs is unknown. Another very difficult question often asked is if the drug would enhance performance or recovery given the amount detected in the urine and whether the substance was unknowingly (via a contaminated supplement) or intentionally ingested.
External Quality Assessment Program
In order to maintain WADA accreditation, each laboratory must comply with the International Standard for Laboratories document (3) and numerous technical documents that address specific issues related to the testing process. Technical documents must be integrated into the policies and procedures of each accredited laboratory and range from compound identification criteria and decision limits for threshold compounds to chain of custody documentation and preparation of documentation packages.
Laboratories must also participate in the WADA external quality assessment scheme (EQAS) and correctly identify prohibited substances in at least 20 unknown samples received throughout the year. Besides blind EQAS samples recognized as the quality assessment samples, laboratories receive double-blind samples indistinguishable from routine samples, which the lab doesn't know are EQAS samples. EQAS samples sometimes contain substances that normally are not identified in routine doping samples. In addition, EQAS samples may contain a low concentration of a prohibited substance as well as more than one prohibited substance. A typical example would be a low concentration of an anabolic steroid together with a diuretic. A false-positive EQAS result earns a laboratory 25 points, whereas they receive 10 points for a false negative EQAS result. For quantitative substances (threshold compounds) a z-score >3 results in 10 points and a z-score >2 but <3 results in 5 points. If a laboratory receives >24 points—meaning just one false positive result—in a single EQAS round, its WADA accreditation will be suspended. When a laboratory receives >30 points over the last 12-month period its accreditation will be either suspended or revoked. This quality assessment program is clearly designed to promote high quality laboratory testing procedures and raises the bar to a very high standard for WADA-accredited laboratories.
The Now and Then
So how big a problem is doping in sports? Of the 267,645 samples tested in 2012 by accredited laboratories, only 1.19% (3,190 samples) were positive for a prohibited substance (9). This low percentage of positivity suggests that either doping is not a significant problem or that doping is really a problem but the cheaters are not getting caught. If one believes the media and recent doping scandals, performance-enhancing drugs are epidemic in sports like cycling and professional baseball. It is more likely that the true story lies somewhere in between: doping is a larger problem than the numbers reveal but is not widespread throughout sports.
Although testing and the associated sanctions for doping violations help deter athletes from using prohibited substances, additional steps need to be taken to win the battle against doping in sports. One approach is to expand unannounced drug testing programs, develop better testing strategies (including longitudinal profiling), and improve current testing methods to catch more, if not all, athletes that dope. Another approach is to provide better athlete education at an earlier age to deliver the message more effectively that doping is unethical and should not be considered or tolerated at any level of sports competition. Lastly, a change in public attitude might be needed. Perhaps a message needs to be delivered to athletes that fans will not continue supporting sports in which doping is perceived to be a major concern.
Anti-doping laboratories face an enormous task in developing and validating testing methods to detect emerging compounds that become available to the sports community. The next major challenge facing laboratories will be to develop analytical methods that detect targeted gene and cell doping. Laboratorians not involved in anti-doping should at least be aware of testing methods that are currently used for detecting prohibited substances and understand new testing modalities as they become available in the fight against doping in sports.
REFERENCES
- Landry GL, Kokotaio PK. Drug screening in athletic settings. Curr Probl Pediatr 1994;24:344–59.
- World Anti-Doping Agency. The world anti-doping code.
- World Anti-Doping Agency. International standard for laboratories.
- World Anti-Doping Agency. Accredited laboratories for doping control analysis.
- World Anti-Doping Agency. The 2013 prohibited list
- World Anti-Doping Agency. Minimum required performance levels for detection and identification of non-threshold substances.
- World Anti-Doping Agency. Identification criteria for qualitative assays incorporating column chromatography and mass spectrometry.
- World Anti-Doping Agency. Laboratory documentation packages.
- World Anti-Doping Agency. 2012 anti-doping testing figures report.
 Anthony W. Butch, PhD, is professor
Anthony W. Butch, PhD, is professor
in the Department of Pathology
and Laboratory Medicine and director of the
Olympic Analytical Laboratory at the
Geffen School of Medicine at the
University of California, Los Angeles,
Los Angeles. Email: [email protected]