Thyroid Screening Recommendations
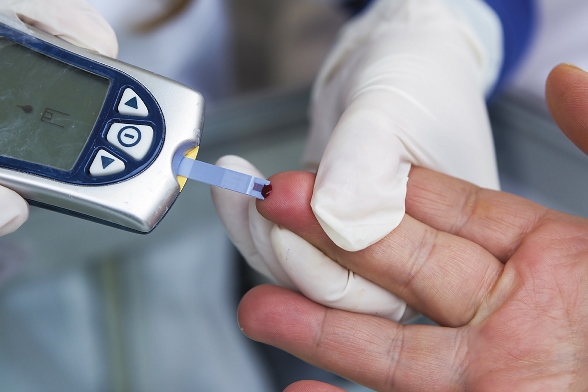
The U.S. Preventive Services Task Force (USPSTF) issued two draft recommendations, one that could boost diabetes screening and another that questions screening for thyroid dysfunction in those without symptoms.
In the first statement, USPSTF said people with risk factors should be screened for diabetes, even if they do not have high blood pressure. The panel signaled it is moving to align with recommendations from the American Diabetes Association, the American Association of Endocrinologists, and others. The draft “B” recommendation suggests screening all adults with risk factors, including being age 45 or older, obesity, a first-degree relative with diabetes, and certain racial and ethnic minorities. Women with a history of gestational diabetes or polycystic ovarian syndrome are also at increased risk. Evidence on the frequency of screening is limited, but the panel suggested that annual screening for those with risk factors may be appropriate. The panel’s previous position from 2008 only recommended screening asymptomatic adults if they had high blood pressure.
The draft recommendation mentions three acceptable screening tests: HbA1c, fasting plasma glucose, and 2-hour oral glucose tolerance testing. It also notes that HbA1c is most convenient for patients, while on the other hand, random blood glucose should not be used.
In the draft recommendation on thyroid screening, USPSTF concluded that current evidence is insufficient to make a recommendation for or against screening for thyroid dysfunction in adults who are not pregnant and show no signs or symptoms of a thyroid problem. This “I” statement indicates the panel believes more research is needed before acting. “People can have mild abnormalities in their thyroid tests and not have symptoms,” said Task Force Co-vice Chair Kirsten Bibbins-Domingo, MD, PhD. “We don’t know enough about the health consequences of finding these individuals and treating them. We need more research in this area.”
With Control of Congress, Republicans Signal Push for Device Tax Repeal
After the midterm elections in November, Republicans will control the Senate and have an increased majority in the House of Representatives in 2015. The day after the election, the Washington Post reported that Senate Republican Leader Mitch McConnell (Kentucky) and House Speaker John Boehner (Ohio) already had drafted a short list of legislation to tackle early in the new year, including a repeal of the medical device tax included in the Affordable Care Act. The medical device tax was levied on diagnostics manufacturers beginning in 2013 and is estimated to cost the industry upwards of $30 billion over 10 years.
Even with their gains, Republicans will not have enough votes to surmount a veto from President Obama. While this makes an outright repeal of the Affordable Care Act that many Republicans have called for unlikely, the Post reported that the two leaders still plan to make changes to the law they think Obama might accept, such as eliminating the Independent Payment Advisory Board (IPAB).
In addition to pushing through changes to the Affordable Care Act, Republicans will also in 2015 chair key committees and subcommittees where the details of healthcare-related legislation get hashed out.
OIG Plans Hard Look at Independent Lab Billing in 2015
Independent labs will be under the microscope for compliance issues in 2015, with a new plan to review Medicare payments, according to the U.S. Department of Health and Human Services (HHS) Office of Inspector General (OIG) Fiscal Year 2015 Work Plan. The plan does not explain in detail which billing practices in particular OIG will target, but notes that OIG will use the results of their reviews to identify clinical laboratories that routinely submit improper claims, and recommend ways for the government to recover any overpayments.
Some clues may come from an OIG report released in July, “Questionable Billing for Medicare Part B Clinical Laboratory Services.” In this report, OIG found that more than 1,000 labs had claims that OIG considered irregular.