A decade ago the first comprehensive guidelines on evaluating and managing chronic kidney disease (CKD) were published, significantly raising awareness of and standardizing definitions for the condition, as well as establishing a common method of reporting kidney function, estimated glomerular filtration rate (eGFR). Since then, considerable research about CKD has taken place and both clinicians and laboratorians have gained ample experience working with CKD terms, definitions, and measurements. Now, a major guideline update published in January 2013 refines CKD risk prediction, highlights important but underappreciated aspects of CKD, and calls upon labs—already important partners in identifying and managing the disease—to embrace a new set of testing recommendations.
"Prior to 2002 we didn't have a classification system for CKD. The 2002 guidelines included a classification system of five stages of CKD based on eGFR, which the lab could estimate from the patient's serum creatinine level, age, and sex. Labs started reporting eGFR, and this classification system has been adopted all around the world. Now we all speak the same language," explained David Wheeler, MD, FRCP, a reader in nephrology at the Centre for Nephrology at University College London. "This new guideline refines the classification system to highlight patients at higher risk of progressing to dialysis or developing cardiovascular complications. The challenge now is to get the guideline disseminated, with the aim of improving patient outcomes worldwide." Wheeler is co-chair of Kidney Disease: Improving Global Outcomes (KDIGO), sponsor of the new guideline, "Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease," which was published as a supplement in Kidney International (Kidney Int Suppl 2013;3).
A New Risk Prediction Model
With some minor changes, the KDIGO guideline kept the definition and staging of CKD first articulated in the landmark 2002 guideline published by the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (KDOQI). That definition—abnormalities of kidney structure or function, present for more than 3 months, with implications for health—was tied to either markers of kidney disease or GFR, with five stages of disease tied to GFR level. The KDIGO work group modified the stages slightly by dividing the old Stage 3 into Stage 3a and 3b, with Stage 3a, mildly-to-moderately decreased GFR, linked to GFR levels 45–59 mL/min/1.73 m2 and Stage 3b, moderately-to-severely decreased GFR, associated with levels 30–44 mL/min/1.73 m2.
The new KDIGO guideline also adds two components, cause and albuminuria, to present a three-dimensional picture of CKD for considering disease prognosis—cause, GFR, and albuminuria (C, G, A). "What the 2002 KDOQI document gave us was a nice simple definition of CKD. Part of the 2013 update by KDIGO is to say we need to make that definition a little more fit-for-purpose and to give physicians a classification system that will allow them to categorize people with kidney disease in the same sort of way that a kidney specialist will do automatically almost without thinking about it," explained Paul Stevens, MB, FRCP, co-chair of the KDIGO work group that produced the guidelines. "A kidney specialist presented with someone with kidney disease will be wondering what their kidney function is, whether and to what degree they have proteinuria or albuminuria, what comorbidities they have, and what the underlying cause is for the disease. So our C-G-A framework recognizes that if you know those three things you can better categorize a given patient's risk." Stevens is a consultant nephrologist and medical director at East Kent Hospitals University NHS Foundation Trust in Canterbury, England.
The C-G-A-based framework for CKD risk prediction also recognizes research advances during the past decade. "There's a growing understanding that more predicts one's prognosis in kidney disease than just creatinine level and eGFR. By defining five stages of CKD the previous guideline kind of lumped them all together. There was a real push-back from the nephrology community that we have all these different diseases, and to combine them into one single category or staging seemed very frustrating and a step backward in advancing understanding of the disease," said panel member Michael Shlipak, MD, MPH. "Before, patients with diabetes-related kidney disease, polycystic kidney disease, or kidney disease that develops as a consequence of aging, all would have been staged the same way, but we know these different causes of kidney disease progress differently." Shlipak is chief of general internal medicine at San Francisco VA Medical Center and professor-in-residence for the departments of medicine, epidemiology, and biostatistics at the University of California, San Francisco.
A Higher Profile for Albuminuria
As with the addition of cause to CKD risk prediction, the work group's decision to use degree of albuminuria both as a component of risk prediction and as the primary test to detect proteinuria reflects an accumulated body of evidence about its utility. "There's been a lot of argument since publication of the original guideline that albumin is a better test of proteinuria and some people disagree with that, but we felt in the guideline group that there was still strong ground for recommending identification of proteinuria using albuminuria testing," said work group member Edmund Lamb, PhD, FRCPath, consultant clinical scientist and head of clinical biochemistry at East Kent Hospitals University NHS Foundation Trust in Canterbury, England. "Further, we felt that by looking at different levels of albuminuria we could risk-stratify patients in the same way it's being done in diabetic nephropathy, extending that classification to all patients with CKD."
The panel endorsed four tests of proteinuria, with urine albumin-to-creatinine (ACR) ratio the preferred measurement (See Box, p. 7). "We recommended albumin corrected for creatinine concentration to adjust for urinary dilution as the primary test for detecting proteinuria because albumin is the most common protein in most nephropathies and there is evidence in diabetic nephropathy that albumin is a strong predictor of progression," explained Lamb. "Further, albumin can be measured using specific quantitative immunoassays down to low levels of albuminuria. Total protein assays are much more susceptible to interferences, have higher variability, and as you're not measuring a single protein, you don't really know what you're detecting. On that basis, we felt that albumin was preferable to total protein." He also noted research findings from the past decade that patients with high levels of albuminuria but normal kidney function nonetheless are at increased risk for acute kidney injury.
|
Recommended Methods of Measuring Proteinuria in Order of Preference
- Urine albumin-to-creatinine ratio
- Urine protein-to-creatinine ratio
- Reagent strip urinalysis for total protein with automated reading
- Reagent strip urinalysis for total protein with manual reading
|
The panel designated reagent strip total protein urinalysis with automated or manual reading as the least desirable methods for proteinuria detection, but kept them on the list because the guideline might be referenced in countries where that type of testing is the most practical, Lamb added.
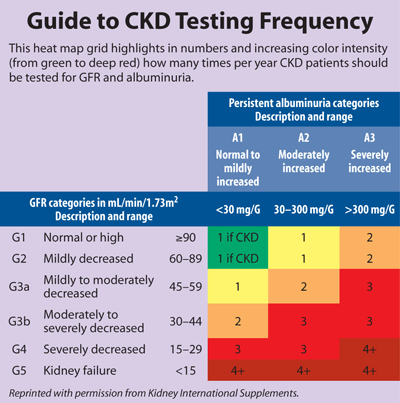
The recommendation to measure albuminuria at least annually for all patients with CKD and more frequently depending on the severity of illness will require retooling by both clinicians and laboratorians, according to Joseph Vassalotti, MD, chief medical officer for the National Kidney Foundation. "In the U.S., Medicare data show that less than 30 percent of patients with diabetes receive recommended annual albuminuria testing. So even in the patients whom everyone agrees would benefit from annual albuminuria screening, it doesn't happen," he observed. "Yet it's very simple and inexpensive and can be done with any office-based visit." Vassalotti did not serve on the panel. (See Table, below, for a recommended testing timeline.)
The End of Microalbuminuria
Labs and clinicians also will need to adapt to the guideline's proposed new terminology for albuminuria, which does away with microalbuminuria in favor of three categories: A1, normal-to-mildly increased, for urine albumin levels <30 mg/G; A2, moderately increased, for levels 30–300 mg/G; and A3, severely increased, for levels >300 mg/G. "Microalbuminuria is a term that produces a lot of misunderstanding, ranging from things you almost find daft that people are looking for, like some small microproteins. It's served its purpose, but it's a term that is outdated now, because we recognize there is a continuum of risk," explained Stevens. "There's a wide range of microalbuminuria, from 30 to 300 mg/G. Telling me I have microalbuminuria doesn't tell me where I am in that range. But telling me I have an albumin-to-creatinine ratio of 35 tells me exactly where I am in that range."
Despite the merits of the new nomenclature, the work group avers that microalbuminuria won't go the way of the dinosaurs overnight. "We discouraged use of the term, but we're fully aware it's embedded in the medical literature and will take more than this guideline to shift people," said Lamb. "But we hope our statement will at least make people realize there's something wrong with the term and perhaps question why it shouldn't be used. Educational programs will hopefully continue to reinforce that message."
The Rising Currency of Cystatin C
Another concept the guideline introduces is the utility of cystatin C in discerning CKD in certain subgroups of patients, particularly those with creatinine-based eGFR 45–59 mL/min/1.73 m2. If the cystatin-based eGFR in these patients also is <60 mL/min/1.73 m2, CKD is confirmed; however, if it is >60 mL/min/1.73 m2, CKD is not confirmed. This suggestion, rather than recommendation, reflects both emerging evidence about this protein, and the degree to which cystatin C testing has been adopted worldwide, according to Shlipak.
"Near the border of 60 mL/min/1.73 m2 creatinine-based eGFR can be inaccurate. More than half of patients with eGFR less than 60 have an eGFR of 45 to 60, and in that group there are a lot of false positives, meaning the eGFR based on creatinine says they have CKD, but their kidney function may be normal. That's where cystatin C comes in," he said. "Beyond just that group of patients, the panelists felt that anytime a clinician is not confident in the eGFR or the stakes are high, for example, when a toxic medication is being dosed or a potentially risky procedure in which kidney function might be impacted, a cystatin C could help to provide a second opinion."
The work group encouraged use of cystatin C even though the test has not been adopted widely and ingrained practices favor use of creatinine rather than cystatin C. "Serum creatinine is part of routine diagnostic sets in the U.S., such as the basic metabolic panel and the comprehensive metabolic panel. Thus, eGFR is routinely reported when the ordering physician is not specifically testing for kidney function," said Vassalotti. "On the other hand, when the clinician orders cystatin C, we can reasonably assume that the indication is confirmatory CKD testing. I'm speculating, but I have the impression that when physicians go to order this test, they hear from the lab that it's an expensive send-out. So the physician may wonder, do I really need this test?"
Shlipak, who has studied cystatin C extensively, echoed the catch-22 that exists right now in which physicians aren't that familiar with the test and many labs don't perform it themselves. However, he stressed that this test should be widely available and preferably performed onsite to speed turnaround times. "Labs should make cystatin C testing available in-house. They should choose an assay that is calibrated directly to the international reference material, and a couple are. Cystatin C, like creatinine, should be reported with an eGFR so that clinicians don't have to learn cystatin C units. All they'll have to do is look at eGFR based on cystatin C," he advised.
The cystatin C dilemma is not unique to the U.S. For example, Lamb and Stevens explained that the test has yet to be recommended by the U.K. National Institute for Health and Clinical Excellence, which develops evidence-based guidelines used to set national health policies, so it is only available in a handful of labs there. However, it is much more common in other European, particularly Scandinavian, countries, according to Lamb.
Taking a Stand for CKD-EPI
The work group also took a stand for reporting eGFR using the 2009 CKD-EPI creatinine equation, putting it somewhat at odds with the National Kidney Disease Education Program, which still recommends the MDRD equation. "One of the controversies that arose after the 2002 guideline was that it was felt that the MDRD equation was underestimating GFR, particularly in people with GFRs in the range 60 to 90 mL/min/1.73 m2, which led to overdiagnosis of CKD. The CKD-EPI equation seems to have some marginal benefits in that particular range. Below 60 mL/min/1.73 m2 one equation probably is as good as the other, although there's evidence accumulating that CKD-EPI is a better risk predictor than MDRD," said Lamb.
The guideline emphasizes that GFR should be measured rather than estimated any time a more accurate GFR will impact treatment decisions. While the work group recognized as the gold standard urinary clearance of inulin during a continuous intravenous infusion, it did not pick favorites among the alternative clearance methods and filtration markers. "The message we were trying to get across was that all estimates of GFR are only an estimate and if you really are concerned that the estimate isn't accurate or if there's a real clinical need to have a more accurate assessment, then you should do a proper GFR," said Lamb. "However, we're not recommending that laboratories do full GFR measurements on everyone because they're time-consuming, labor-intensive, expensive tests. So it should be reserved for cases where there are doubts. Among the GFR reference methods, we weren't recommending one over the other. They all have their good and bad points, and practices differ around the world." (See Table, below)
|
Pros and Cons of Glomerular Filtration Rate Methods and Markers
|
| Method |
Pros |
Cons |
|
Urinary Clearance
Bladder catheter and continuous infusion of marker
|
Gold standard |
Invasive |
Urinary Clearance
Spontaneous bladder emptying |
Patient comfort |
Low flow rates in patients with low GFR |
Urinary Clearance
24-hour urine collection |
|
Cumbersome; prone to error |
| Plasma Clearance |
Potential for better precision |
Overestimates GFR in extracellular volume expansion |
| Nuclear Imaging |
No urine collection or repeated blood sampling |
Less accurate |
|
Markers
|
Pros
|
Cons
|
| Inulin |
Gold standard |
Expensive; difficult to dissolve and maintain in solution |
| Creatinine |
Assay available in all clinical labs |
Intra- and inter-individual secretion varies |
| Iothalamate |
Inexpensive; long half-life |
Handling, storage issues when 125I used as tracer; inappropriate in patients with iodine allergy |
| Iohexol |
Inexpensive |
Inappropriate in patients with iodine allergy; use of low dose requires expensive assay |
| EDTA |
Widely available in Europe |
Handling, storage issues when 51Cr used as tracer |
| DTPA |
Widely available in U.S. |
Handling, storage issues when 99mTc used as tracer |
|
Abbreviations: EDTA, ethylenedianine tetraacetic acid; DTPA, diethylenetriamine pentaacetic acid.
Adapted from Kidney Int Suppl 2013;3:55.
|
The Less-Appreciated Aspects of CKD
The guideline also emphasizes aspects of CKD management that arguably have received short shrift in the past, such as considering the impact on renal function when choosing medications and patient safety during procedures. "The guideline really speaks to recognition that CKD is a patient safety risk. For example, two-thirds of prescription drugs are cleared by the kidney, so obviously when the patient has impaired kidney function clinicians should modify what they prescribe based on the level of kidney function," said Vassalotti. "Also, contrast agents used in diagnostic imaging can be potentially nephrotoxic, underscoring the importance of a patient safety approach that considers the level of kidney function."
In support of messages labs have been sending for some time, the guideline stresses that cardiac tests in CKD patients, including B-type natriuretic peptide (BNP), N-terminal pro-BNP, and cardiac troponins, need to be interpreted cautiously. The work group also recommended measuring serum levels of calcium, phosphate, parathyroid hormone, and alkaline phosphatase activity at least once in adults with GFR <45 mL/min/1.73 m2. In patients with GFR <45 mL/min/1.73 m2, the panel recommended maintaining serum phosphate concentrations in the normal range.
All the experts who spoke with CLN emphasized how crucial labs have been in raising awareness of and improving care for patients with CKD, and they expressed confidence that lab professionals would again play an important role in disseminating and adopting the KDIGO guidelines. However, they also noted how much ground remains to be covered in the field, both in reaching patients with CKD and in advancing evidence where knowledge gaps exist. "Because kidney disease is mostly asymptomatic, most patients feel well. U.S. NHANES survey data indicate that among those with eGFR <30 mL/min/1.73 m2, only about 42 percent are aware of their condition," said Vassalotti. "The lab is the most important place where diagnostic testing for CKD is performed, and clinical laboratory professionals have already and will continue to make a huge difference in kidney disease by adopting this guideline as much as feasible within their local individual practice context."