CDC Updates HCV Testing Algorithm
In response to data showing only about half of those identified as having hepatitis C (HCV) ever received a follow-up RNA test, the Centers for Disease Control and Prevention (CDC) is updating its HCV testing guidelines to recommend nucleic acid testing (NAT) for HCV RNA on all antibody-positive results.
The new testing guidelines are based on a CDC Vital Signs study on HCV in which researchers looked at data from eight areas across the nation. Of the HCV cases reported in these areas with antibody-positive results, only 51% also included a follow-up RNA test to identify current infection. Without RNA testing, the other half are likely unaware if they are currently infected and therefore do not get appropriate medical care, CDC noted. A small number of people with antibody-positive tests will have cleared the infection on their own, but most people with HCV—about 80%—will remain infected and can go on to develop significant health problems.
CDC is also focusing on follow-up RNA testing because of developments in testing technology and significant advances in antiretroviral agents effective against HCV, the agency said.
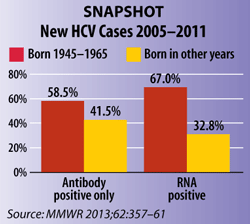
Data in the Vital Signs analysis underscoring the severe impact of HCV on baby boomers also motivated the change. In the eight areas studied, 67% of all reported cases of current infection were among those born from 1945 through 1965. Nearly three-quarters of all deaths among people with HCV also occurred among baby boomers.
The new testing recommendations call for a lab-based HCV antibody test or rapid point-of-care test. For lab-based assays, it is not necessary to report signal-to-cutoff ratios as in the past. CDC recommends that in cases where it is necessary to distinguish between true positivity and biologic false positivity for an HCV antibody test, a second, different FDA-approved antibody assay should be used. Because HCV antibody assays differ in antigens, test platforms, and performance characteristics, specimens are unlikely to yield false-positive results more than once.