
As the healthcare field continues to navigate uncertain and difficult terrain, companies that provide new tools for clinical labs must move swiftly to stay on top of emerging technologies and the latest clinical developments. The 2013 Clinical Lab Expo showcases the industry at its best, as companies compete to provide innovative solutions to clinical lab scientists who are passionate about quality and value. The expo opens today with nearly 700 companies filling more than 200,000 square feet of exhibit space in the George R. Brown Convention Center. In addition, more than 100 new exhibitors from around the globe have chosen this year's Expo to make their debut at this must-attend event.
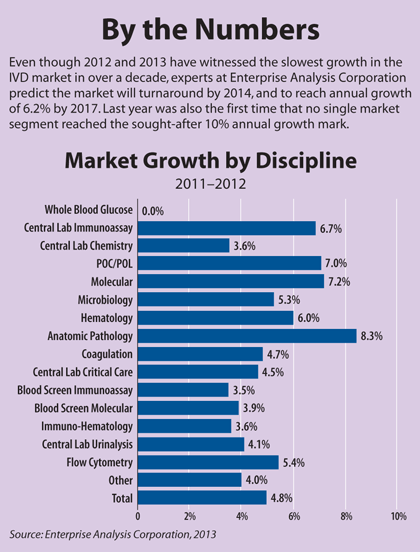
Market reports show that the past several years have not been easy for the IVD industry. A lingering global recession and pressure on reimbursement have taken their toll. On a global scale, IVD industry sales grew at an anemic 4.8% last year, much lower than the 6% annual growth seen before the recession peaked in 2008, according to a report from the market research and consulting firm Enterprise Analysis Corporation (EAC). However, despite slower growth in 2012 and 2013, experts still remain optimistic that the market by 2017 will climb near the $70 billion mark in annual sales.
"We think the market will gradually rebound over the next five years, possibly reaching a six percent annual growth rate by 2017," said EAC Vice President Gerard Conti. "However, the emerging markets, such as China and India, will represent a bigger piece of the overall IVD pie. Compared to North America and Europe, they will be driving more of the overall growth than in the past."
Healthcare Reform in 2014: Boon or Bust?
Since Congress passed the Patient Protection and Affordable Care Act in 2010, the U.S. IVD industry and other healthcare sectors have had one date on their minds—January 1, 2014. In fewer than 6 months, possibly the most significant element of the law will come into effect as millions of uninsured Americans enter new insurance exchanges and expanded Medicaid programs. The big question for healthcare companies has been, will some 30 million newly insured people drive higher volumes of testing and other services?
According to analysts at EAC, a rise in the number of insured in the U.S. will likely be compounded with other factors to revive the IVD market: a recovering global economy, an aging population in developed countries, and a rising middle class in China, Brazil, and other nations will combine to lift volumes and sales.
IVD companies are not the only ones looking to 2014 to boost volumes. Commercial labs, such as Quest and LabCorp, have been struggling over the last few years with nearly flat organic volume growth, noted Kevin Ellich, a senior research analyst for investment bank Piper Jaffray. "Management of the independent labs still believe they will get back to the two to four percent annual volume growth they experienced in the past," Ellich said. "But this brings us back to the question many in the healthcare industry have been asking, whether we are really in a new normal environment where volumes and reimbursement remain pressured. Right now, it looks like the saving grace could be healthcare reform."
It's not certain that expanded insurance under the Affordable Care Act will boost volumes, but Ellich expects at least some effect. "I think there will be some positive impact on testing volumes. My belief is that of the 30 million new covered lives, some subset were not accessing the healthcare system, so there should be some incremental increase in testing volume. But we'll have to wait and see what the overall impact on revenues and profitability will be for labs." About 70% to 80% of the bad debt independent labs carry comes from the uninsured, Ellich noted, though it's a small part of overall testing.
At the same time, several elements of the Affordable Care Act that have been implemented already could counterbalance this volume effect, Ellich said. "An emphasis on overutilization of testing and treatments, as well as a shift to a bundled payment model and accountable care organizations, could take away incentives that existed for unnecessary testing, and this will have an impact on volumes," he said.
A report published in June by the consulting firm PricewaterhouseCoopers predicts that price inflation in healthcare will continue to drop in 2014, down to 6.5%, as a result of new models and new venues for providing care. The report calls out four factors that will slow the growth of medical costs in 2014: care moving out of hospitals into retail clinics or mobile health; large employers such as Walmart contracting directly with big-name health systems; federal government readmission penalties; and the growth of high-deductible health plans that make consumers much more cost-conscious.
What do these trends mean for the healthcare marketplace? PricewaterhouseCoopers recommends a focus on consumers and value. "Employer engagement and individual consumers are a powerful and growing force in the health ecosystem. To succeed, healthcare organizations should fashion strategies around new demands for value," the report authors concluded.
Price Pressure, Competition Shift the Market
Over the past two decades, the diabetes testing market was a huge growth driver, with double-digit growth almost every year. As prices have dropped for blood glucose test strips, and more competitors have entered the market, growth in this segment has bottomed out. As whole blood glucose testing flattened, companies turned to higher-priced molecular tests to lead growth, where growth hovered around 10% each year. Now, molecular has dropped below 8%.
EAC's Vice President Mark Hughes noted that even as companies have developed new molecular testing technology, reimbursement is now emerging as a problem, compounded by a slow economy. "We often see reports in industry newsletters of molecular diagnostics still growing at fifteen or eighteen percent, but we don't buy it," Hughes said. "We perform a careful, bottoms-up, company-by-company review of revenues, and when we put that all together, it's not even ten percent" (See Box, below).
While EAC is predicting that the molecular segment will reach a compound annual growth rate of about 8% between 2012–2017, uncertainty over reimbursement in the U.S. as the government shifts to a new coding scheme will not make it an easy path. This year, the Centers for Medicare and Medicaid Services (CMS) announced that new reimbursement codes for molecular tests would not carry over the payment associated with the old "code stacks" to which labs had grown accustomed. Now, local administrative contractors have developed their own pricing schemes and submitted them to CMS, which plans to take the average of the regional prices as the maximum reimbursement under Medicare for 2014.
"We don't have all the company data yet on this ongoing issue, but my inclination is that the new molecular codes will have an overall negative impact," Hughes said. "It will also make it more difficult for IVD companies to persuade laboratories performing lab-developed tests to convert to FDA-approved kits if reimbursement is cut significantly."
In addition, EAC predicts greater competition in molecular testing—increasing the choices for labs, but likely tamping down prices and reducing revenue for IVD companies. Particularly in point-of-care testing, there are dozens of companies now which plan to roll out molecular platforms, noted Conti.
Commercial labs not focused exclusively on molecular tests will be somewhat cushioned from this blow, according to Ellich. "For companies like Quest and LabCorp, molecular testing accounts for about five to ten percent of total revenues, so it's not as material as routine clinical testing," he said "That said, Medicare's proposed molecular reimbursement rates that came out in May indicate that the cut in 2014 will be about 16 percent on average by our calculation, which is still a significant number, but it is better than the initial 20 to 25 percent cut in 2013."