What if it took months of tedious work for your new mp3 player or printer to connect to your home computer? In the world of consumer electronics, such a delay would spell the end of any new product. In fact, consumers expect instantaneous connections. Labs, on the other hand, often wait up to 6 months and spend thousands of dollars before a new instrument will reliably connect with their lab information system (LIS). The cost, downtime, and inevitable human errors associated with bridging the many gaps in electronic communication have beset labs for decades. But the good news is that help will arrive soon.
After 5 years of work, a group of instrument manufacturers and software developers called the In Vitro Diagnostics Connectivity Consortium (IICC) has successfully tested a new standard for lab connectivity. Once finalized, the standard will enable rapid and uniform connections among a variety of elements in labs that depend on information technology (IT), including clinical chemistry, immunoassay, molecular and other analyzers, the LIS, automation systems, middleware, and electronic health records (EHR). The IICC also crafted the standard with an eye towards paving the way for better integration with IT systems of the future, a challenge laboratorians already know will put significant demands on the lab.
"The current lack of standardization hurts everybody, and increases the costs for labs as well as vendors," said IICC president Eric Olson. "Both labs and vendors spend a great deal of time and money customizing the interfaces for each instrument. In our view, that time and money would be better spent elsewhere. The IICC connectivity standard will enable labs and vendors to focus on innovation that pushes the industry forward, as opposed to just figuring out how to make the technology we have now work together." Olson is vice president of portfolio and product management at Siemens Healthcare Diagnostics.
Getting to Plug and Play
While the lab has historically been a leader in IT, adopting electronic systems well before other disciplines in healthcare, standards for instrument-to-LIS communication have fallen behind. The current standards written by the American Society for Testing and Materials (ASTM) date back to the 1980s. The Clinical and Laboratory Standards Institute (CLSI) took ownership of the standards in 2001, now known as LIS-1 and LIS-2.
ASTM designed the old standards to be flexible in anticipation of new technology. However, this very flexibility created a situation where each instrument and IT vendor interpreted them in a slightly different way, Olson explained. As a result, each new instrument—or instrument feature—that a lab wants to connect to its LIS requires a special package of code called a driver that can translate data between the LIS and the instrument. Making these interfaces work can cost $5,000–$10,000 for each new instrument, and even more for an automation line—not to mention the extra weeks or months it takes to get the whole system connected properly, and additional maintenance and troubleshooting when IT problems arise (See Figure, below).
How Lab Connectivity Works Today
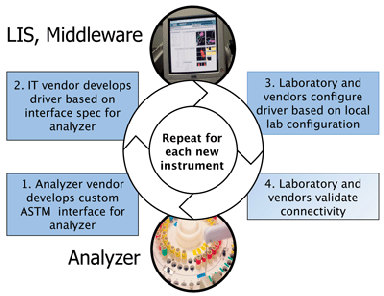
Under current connectivity standards, each vendor tends to interpret messages a little differently, requiring laboratory information system (LIS) companies to write code that translates between each unique system. Under a new uniform standard developed by the IVD Industry Connectivity Consortium, instruments and software will achieve plug and play connectivity.
According to Scott Goss, general manager of Abbott's OneLab, the company's data management and analytics system, manufacturers are just as eager to solve these problems as their lab-based customers. "As vendors try to work out how instruments and software can speak together, customers have been caught in the middle," he said. "Our goal and their goal is to get a system up and running as soon as possible, but developing these interfaces often results in significant delays." Goss serves on IICC's executive committee as treasurer and secretary.
For instrument manufacturers, simply tweaking the code in their interfaces is not really an option, explained Rob Bush, president of Orchard Software, an LIS developer, and a member of IICC's Technical Leadership Team. "Instrument manufacturers write their interface, test it the best they can, and submit the whole thing to the Food and Drug Administration (FDA) to get approval, only to discover later that the way they interpreted the standard for their interface was a little different than everyone else's," Bush said. "But their code might as well be set in concrete, because they've submitted it to the FDA and if they want to change it, they have to go submit it again." For Orchard and other software companies, this requires writing special new code for their LIS to work with a new instrument. Orchard, for example, has developed some 50 different unique communication protocols.
The persistent and expensive problems caused by the old standards explain the enthusiasm surrounding a successful test of IICC's new, uniform standard. The new standard was put to the test this year in Berne, Switzerland at a special meeting called a connectathon. At the May event, instrument vendors brought virtual versions of their instruments and LIS vendors came with their systems, and together they performed real-world testing of communication protocols. In developing the standard, IICC partnered with an organization experienced at developing connectivity standards, Integrating the Healthcare Enterprise (IHE). Consequently, the new IICC standard is called IHE-LAW, for laboratory analytical workflow.
"The connectathon is like a round robin where each dance partner dances with every one of the others to see if they can communicate," Olson said. "All of the vendors' engineers were there on site, so by the end of the connectathon, all of the developers were able to straighten out any issues where there were different interpretations of the standard such that we had 100 percent test completion by the end of the connectathon. This is one of the remarkable outcomes, that each of the IT systems tested used a single driver to communicate with each of the different instruments, because the instruments actually spoke the same language."
Once IHE-LAW is finalized and adopted by instrument and IT manufacturers, which is anticipated to occur sometime in 2013, there will no longer be special interfaces for each new analyzer. Instead of implementing a different driver for each instrument, IT companies will implement a single driver that works with all instruments that are IHE-LAW compliant.

Seven leading in vitro diagnostics vendors gathered in Berne,
Switzerland in May to test a new instrument-LIS connectivity standard
from the IVD Industry Connectivity Consortium. By the end of the event,
each instrument interface was able to connect with each laboratory information system
without a driver—a costly piece of special software that labs currently need
to translate between instruments and the LIS.
Speaking a Universal Healthcare Language
Perhaps the most significant shift that will occur with adoption of the IHE-LAW standard will be its basis in HL7, a global electronic messaging language recognized by the International Organization for Standardization (ISO). Because HL7 bears ISO's imprimatur, it has become the most widely implemented healthcare standard in the world for exchanging clinical data between systems.
Basing the IHE-LAW on HL7 should allow for more seamless integration of lab data with EHRs, information exchanges, and other large-scale IT systems that share an HL7 superstructure. However, the task for IICC is not as simple as just adopting HL7. To ensure that instruments and LISs use HL7 in a uniform way, the IHE-LAW standard sets forth exactly how instruments should implement it, Bush explained. "Agreeing that we're going to do an HL7 interface is a little bit like saying that we're all going to speak English. So for example, if each of us wrote a paragraph or a book describing what we do, our sentences and syntax are not going to be identical."
The implementation of HL7 allows for a huge range of interpretations, Olson noted. "HL7 has many fields where you can put all kinds of information, and it's really up to the vendor how to use those fields. So just using HL7 doesn't get us anywhere near plug and play," he said. "IHE-LAW is really about constraining things such that when you have a certain transaction, for example sending an order to an instrument or getting a result back from an instrument, it's very prescriptive as far as what data you have to put in each field, how you format that information, and how that whole transaction needs to take place—all things that the communicating parties need to agree on."
"Driving the lab industry toward HL7 opens up a lot of new opportunities for better interaction with other IT systems. Labs should expect to become more deeply integrated into EHRs and other enterprise-wide systems," explained Jay Jones, PhD, who directs the regional laboratories at Geisinger Health System in Danville, Pa. "IT in general is going very global, so we need to get beyond the lab instruments talking in their own parochial dialect. If the lab is going to be successful in supporting front-end decision support, computerized physician order entry (CPOE), and connecting with a variety of different systems, we will need HL7 and more modern connectivity standards like IHE-LAW to make that happen." Jones serves as the provider review committee chair for IICC.
Hospitals Demand Quality Data
In addition to the benefits that directly affect lab operations, adoption of the new standard also promises to enhance the quality and consistency of data labs share with EHRs and other programs. With current instruments all speaking their own, unique language, they also produce data in a variety of formats, making data less comparable from instrument to instrument and from lab to lab. "The idea of IHE-LAW is that we're working on the standards that reside deep down in the instruments that produce data at its source," Olson said. "If we can get uniform data, all formatted the same way, using the same fields coming out of the instruments, then labs have a clean and consistent data stream that feeds up into the EHR. We want to improve data quality by cleaning it up at the source, rather than trying to do so retrospectively."
Jones emphasized that the government's EHR incentive program will require more and more from labs. Under the program, providers such as hospitals and physicians must demonstrate meaningful use of EHRs to receive financial incentives, and more than 80% of hospitals say they expect to take advantage of incentives for EHR meaningful use by 2015. By 2014, providers intending to demonstrate meaningful use must employ CPOE and decision support, both of which will rely on rapid and dependable exchanges of lab data.
"Meaningful use will require a lot of real-time, fluid message sharing between lab systems and other systems. I think a prime example is what is happening with cardiac markers in emergency departments. Patients will need to have a lab result back within one hour of presentation at the emergency department," Jones said. "Meaningful use will also require that a certain percentage of lab orders come through the EHR, which are all based on HL7, so there again the message format will be very important. In some cases, we may even see reflexive orders come directly from the instrument, which is allowed in the IHE-LAW framework."
Powering Next-Generation Instruments
In some respects, IHE-LAW aims to get labs up-to-date with the rest of healthcare. However, the software companies and instrument manufacturers supporting the new standard are thinking about the future as well.
As EHRs and CPOE become more established forces in healthcare, traditional models of how orders and results flow through the lab will have to change, Jones noted. "Right now we have a lab model of orders and results locked in a certain path: the physician generates an order, the order comes to the LIS, the LIS spots the tube with the label on the instrument, uploads the test order, the instrument does the test, and results come to the LIS, then out to the physician," he said. "But I think a lot of that is going to become more complex when we have expectations of orders and results happening real-time through CPOE and point-of-care instruments."
Both instrument manufacturers and LIS vendors now expect that instruments themselves will have to get smarter, in some cases taking orders directly, or even employing rules for reflex testing and decision support. But if instruments take on these new roles, their communication standards will have to evolve, too. In a pre-IHE-LAW world, many innovations have hit a brick wall, Bush explained.
"Some instrument vendors have tried to make their instruments more competitive by adding decision support and reflex testing. In order to do that they need more information from the LIS, or they need to work in concert with LIS capabilities," Bush said. "However, without a uniform connectivity standard, these advanced features often just won't work, even with additional code in the interfaces, and I think this has been a disincentive for instrument manufacturers to push the envelope."
The new IHE-LAW standard will allow instruments to take on some of these roles, giving the LIS and instruments the ability to exchange more information about the patient or access delta check data—and potentially even to grab information from another analyzer for reflex testing. With a new standard that enables LISs and instruments to trade this information back and forth, companies can feel confident about pushing the envelope with new features. "The new standard allows us to support roles for LIS and equipment in the laboratory that are much more dynamic and flexible than we've ever been able to do in the past," Bush said. "Middleware will also benefit from the expanded information available. Having more robust interfacing capabilities will make the use of middleware even more attractive."
If many of IICC's strategies seem reminiscent of recently adopted standards for point-of-care (POC) instruments, it's because IICC modeled much of its work on IHE-LAW on the POC connectivity standard finalized in 2001. "In some ways, we're seeing the central lab instruments catching up to where point-of-care already is," Jones said. "But there is already talk about it all being so transparent that the standards merge, so that the CLSI point-of-care standards and the future CLSI central lab IHE-LAW standards are one and the same."
Getting Ready for the Change
IICC has drafted and tested the IHE-LAW standard, but a few steps remain. IICC plans to further refine IHE-LAW with more testing at another connectathon event in 2012. The goal is that by the end of 2013, IICC can work with CLSI on publishing a final, approved standard. However, Olson is encouraging lab industry engineers to begin working with the draft standard immediately.
"It is of great advantage to vendors to get started now, since we really are just talking about tweaks to the standard going forward," Olson said. "Really, the biggest change is going to be moving to HL7. Training engineering staff on how to use HL7 is another big step. So we will make as much technical information available as possible early on so that vendors have time to get up to speed such that when we have this final standard, the vendors all know what to do with it."
Already, some of the top instrument manufacturers have signed on with IICC to support the new standard, including Abbott, BD, Beckman Coulter, bioMérieux, Ortho Clinical Diagnostics, Roche, and Siemens. Participating LIS vendors include Data Innovations, Orchard Software, and Systelab.
"By coming together on this standard we will be able to improve the overall delivery of care," said Goss. "Now we are focused on reaching out to drive adoption of the standard by both LIS and instrument vendors."