Hepatitis C virus (HCV) infection has the dubious distinction of being the most common chronic blood-borne infection in the U.S., with an estimated 3.2 million people chronically infected. With these staggering numbers, HCV infection has become the most frequent indication for liver transplant in the U.S. (1). HCV is also a global problem, with 170–200 million people infected worldwide.
Projections based on the current prevalence of infection and anticipated rates of progression have raised concerns about the potential impact of the virus during the next 2 decades. A computer cohort simulation of the U.S. population suggests that as the duration of infection increases in the surviving cohort, the proportion with cirrhosis in an untreated population will increase from 16% in 2000 to 32% by 2020. Complications of cirrhosis, including hepatic decompensation, hepatocellular carcinoma, and liver-related deaths, will also increase dramatically during the next 20 years (2).
Improvements in diagnosis and disease management, including newer direct-acting agents (DAAs), have the potential to alter this trend. Although several vaccines are currently under development, with some already in Phase I/II human clinical trials (3), no protective vaccine is presently available for HCV. Developing an effective vaccine has been challenging because the virus mutates frequently, thereby evading immunologic surveillance.
Until the tide of this disease can be altered, diagnosing active HCV infection will be crucial to slowing its spread. Here we discuss laboratory tests used for screening for the disease, as well as for patient management.
Transmission and Risk Factors
HCV is transmitted primarily through direct percutaneous exposures to blood containing HCV. In the U.S., blood transfusions and intravenous drug use (IVDU) are the two most common exposures associated with transmission. While blood transfusions accounted for a substantial proportion of HCV infections acquired 25 years ago, that is no longer the case. Today, the risk of transfusion-associated HCV infection is extremely low, but it is not zero. In contrast, IVDU consistently has accounted for a substantial proportion of HCV infections and currently is the cause of about 60% of HCV transmission in this country.
Physical Characteristics of HCV
HCV is a member of the genus Flaviviridae. Its genetic material, or genome, consists of a single strand of RNA approximately 9,400 nucleotides long that encodes six to eight proteins (Figure 1). Two non-coding regions, referred to as the 5'-untranslated region (UTR) and the 3'-UTR, flank the protein-coding regions of the genome. The 5'-UTR is the main target detected in nucleic acid-based assays, while serologic assays detect HCV antibodies directed against peptides c22-3, c33c, and c100-3 that are derived from the various viral proteins.
Figure 1HCV Genome
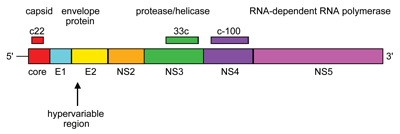 HCV is a single-stranded RNA virus with a genome of approximately 9,400 nucleotides that encodes six to eight proteins.
HCV is a single-stranded RNA virus with a genome of approximately 9,400 nucleotides that encodes six to eight proteins.
Different strains of HCV show a considerable degree of nucleotide sequence diversity. By comparing the nucleotide sequences of different strains of HCV, the virus can be divided into six main groups or genotypes and more than 50 subtypes (4). The clinical implications of these genotypes are discussed below.
Screening and Testing
Individuals with specific risk factors should be screened for HCV. These risk factors include: illicit drugs injection, even if only one time; conditions associated with HIV infection; hemodialysis; unexplained high aminotransferase levels; and blood transfusions and/or organ transplants prior to July 1992. Furthermore, children born to HCV-infected mothers, as well as healthcare, emergency medical, and public safety workers who may have been exposed to HCV-positive blood from a needle stick injury or mucosal exposure should be screened (5). In August 2012, the Centers for Disease Control and Prevention (CDC) added persons born from 1945 through 1965 to the list of at-risk groups, expanding its 1998 guidance on prevention and control of HCV infection and related chronic disease (6).
Figure 2 shows CDC's recommended testing algorithm for anti-HCV screening assays that use serologic assays to detect specific antibodies to HCV to diagnose HCV infection.
Figure 2CDC Recommended Testing Algorithms
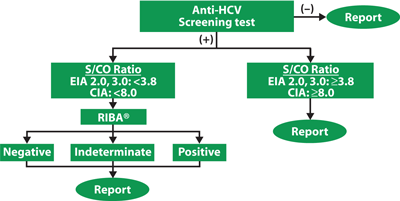 Labs should follow CDC's comprehensive recommendations for reporting results of anti-HCV testing according to the types of reflex supplemental testing performed (8).
Abbreviations: S/CO: signal-to-cutoff ratio; EIA: enzyme immunoassay; CIA: chemiluminescent immunoassay; and RIBA: recombinant immunoblot assay.
Labs should follow CDC's comprehensive recommendations for reporting results of anti-HCV testing according to the types of reflex supplemental testing performed (8).
Abbreviations: S/CO: signal-to-cutoff ratio; EIA: enzyme immunoassay; CIA: chemiluminescent immunoassay; and RIBA: recombinant immunoblot assay.
Today's enzyme immunoassays (EIAs) and chemiluminescent immunoassays (CIAs) have >99% specificity for HCV antibodies. Current HCV nucleic acid tests (NATs), also referred to as HCV RNA tests, as well as transcription-mediated amplification assays, also have excellent specificity for detection of HCV in plasma or serum. FDA-approved HCV NATs should be used to identify active HCV infection among persons who have tested positive for HCV antibodies.
CDC recommends HCV genotyping for all HCV-infected persons prior to initiating interferon-based treatment. This information allows the clinician to plan the patient's dose and duration of therapy, as well as estimate the likelihood of response. Genotype 1, which is more prevalent in the U.S., has lower response rates compared to genotypes 2 and 3 and also requires longer treatment. For some patients, IL28B genotyping is used as a pretreatment predictor for sustained virological response (SVR) to either combination therapy or triple therapy in patients with genotype 1 chronic HCV infection (7).
Signal-to-Cutoff Ratios
As noted in Figure 2, confirmation of reactive screening results with supplemental testing such as the recombinant immunoblot assay (RIBA) is not always necessary. Analysis of early versions of anti-HCV EIAs on samples from volunteer blood donors indicated that on average, repeatedly reactive signal-to-cutoff (s/co) ratios could be used to predict supplemental test-positive results (8). Subsequent studies and additional CDC data on other populations confirmed that a specific s/co ratio predicts a true antibody-positive result ≥95% of the time, regardless of the anti-HCV prevalence or characteristics of the population being tested. For EIA, a screening-test-positive average s/co ratio ≥3.8 was highly predictive of RIBA positivity (≥95%). The range of screening-test-positive s/co ratio by CIA, however, was greater than by EIA; therefore, most labs use a s/co ratio ≥8.0. Figure 2 also indicates how labs should report HCV testing results.
Liver Biopsy
In general, physicians treat all their patients with chronic HCV infection either without evaluating a liver biopsy or by customizing treatment based on histologic findings and the probable length of time that the patient has had the infection. Liver biopsy provides helpful information on liver injury status and gives physicians other information that is useful for making decisions about follow-up and therapy. For example, the results also may reveal advanced liver fibrosis or cirrhosis that requires surveillance for hepatocellular carcinoma and/or screening for varices.
Biopsy results are less than ideal, however, as the procedure samples only a very small amount of liver tissue and the biopsy material may not represent the most severe changes in the liver. In addition, liver biopsy carries a small but real risk of significant complications, such as hemorrhage and bile peritonitis.
To date, liver biopsy has been the gold standard for evaluating HCV infection, but that standard is now being challenged. Already, available non-invasive testing technologies and other technologies currently being evaluated are poised to someday replace liver biopsy.
Treatment Protocols
Various treatment protocols have been established for chronic HCV infection that incorporate interferon, pegylated interferon, and ribavirin in various combinations. These treatments are referred to as mono- and combo-therapies (See link to Table 2, below). In 2011, FDA approved two protease inhibitors, boceprevir (Victrelis) and telaprevir (Incivek), for treating HCV genotype 1 infection. These new DAAs represent a significant advance in treating chronic disease and may change care standards. Adding one of the drugs to combination therapy achieves a significant increase in sustained viral response (9) and is called triple therapy.
Predictors of Treatment Outcome
Table 1 lists predictors of HCV treatment response. Molecular factors associated with response are summarized in more detail below.
|
Table 1
Predictors of HCV Treatment Response
|
| Factor |
Favorable |
Unfavorable |
| Baseline Viral Load |
<800,000 IU/mL |
≥800,000 IU/mL |
| HCV Genotype |
2,3 |
1,4,5,6 |
| Cirrhosis |
Absence |
Bridging, cirrhosis |
| Age |
<40 |
>40 |
| Gender |
Female |
Male |
| Body Mass Index |
<28 |
>28 |
Relative increase in ALT
(x upper reference limit) |
≥3 |
<3 |
| Ethnic Origin (IL28B) |
European, Hispanic |
African |
Baseline Viral Load. A well-recognized predictor of treatment outcome, a baseline viral load of <800,000 IU/mL, has been associated with a more favorable response to therapy (10). In addition, quantitative assessment of serum HCV RNA levels allows physicians to identify those patients with low levels of HCV RNA who may have a greater chance of achieving a durable response to treatment. Physicians generally measure viral load prior to initiating treatment because knowing the magnitude of HCV infection is helpful for discussing the risks and benefits of therapy with the patient. In addition, it also provides a baseline for determining the extent of viral load decline during treatment.
HCV Genotyping. Various studies have suggested that baseline viral load might not be the only factor influencing the response to interferon treatment. In fact, several have shown that HCV genotypes differ significantly in their response to treatment (10, 11). The reasons for these differences are unclear, but subtypes of HCV may have different replication efficiencies, giving rise to different serum levels of virus and responses to interferon. Alternatively, research suggests that there may be a genetic basis for viral resistance to interferon. In general, patients infected with genotypes 1, 4, and 5 tend to show less response to interferon therapy than those infected with genotypes 2 and 3.
IL28B Genotyping. In addition to viral genotype, host genetic factors also appear to play a role in the course of infection. Recent studies have identified a specific polymorphism near the gene for lambda interferon 3 on chromosome 19 (IL28B) as having a marked effect on spontaneous clearance of virus. Studies show that the CC genotype at position rs12979860 is twice as common in persons who spontaneously clear HCV infection compared to those who have progressed to chronic HCV.
The IL28B polymorphism also appears to influence treatment response. In fact, studies have shown that the predictive value of the IL28B genotype for SVR is superior to that of several other variables, including baseline HCV RNA level, fibrosis stage, age, and gender, and that it also is higher for HCV genotype 1 than for genotypes 2 and 3. Other polymorphisms near IL28B also predict SVR, including rs8099917.
Monitoring Treatment
For patients receiving combo-therapy, labs should be careful to assess HCV RNA levels before therapy is started and after 12 weeks of therapy using the same quantitative assay. An early virological response (EVR), defined as a ≥2 log reduction in HCV RNA levels during the first 12 weeks of therapy, is a valuable clinical achievement. In the absence of an EVR, the likelihood of a SVR is >3%. When the only goal of therapy is to achieve SVR, the physician may choose to discontinue therapy after 12 weeks if there is no EVR. Histologic benefit may occur even in the absence of SVR; therefore, some clinicians treat beyond 12 weeks even without an EVR.
Table 2
Treatment Protocols Using Various Dosing Regimens for Chronic Hepatitis C
| |
Treatment
|
Recommended Dose
|
Treatment Duration
|
| Mono |
Interferon α-2b
Interferon α-2a
Interferon alfacon-1 |
6 MIU t.i.w.
3 MIU t.i.w.
9 µg t.i.w. |
48 weeks
72 weeks
24 – 48 weeks |
| Combo |
Interferon α-2b +
Ribavirin
PEG-IFN +
Ribavirin
|
3 MIU t.i.w.
1,000 – 1,200 mg daily*
180 µg or 1.5 µg/kg weekly
1,000 – 1,200 mg daily*
|
24 weeks – Relapse
24 – 48 weeks – Naïve
24 weeks - Genotypes 2/3
48 weeks – Genotype 1
|
| Triple |
PEG-INF + RBV + Telaprevir
PEG-INF + RBV + Boceprevir |
TVR: 750 mg t.i.d.
BOC: 800 mg t.i.d. |
12 wks triple + 36 wks combo
4 wks combo + 32 wks triple + 12 wks combo |
|
Abbreviations: MIU=million international units; t.i.w.=three times weekly; t.i.d.=thee times daily
*Dosage based on body weight: patients weighing < than or equal to 75 kg receive 1,000 mg oral ribavirin, patients weighing >75 kg receive 1,200 mg oral ribavirin. FDA approved 800 mg daily dose of ribavirin when used with PEG-IFN, especially in patients who weigh <65 kg.
|
Documenting the patient's response at the end of therapy or SVR at >6 months after completing therapy requires a more sensitive assay with a low detection limit (12). Labs should be careful to select an HCV RNA assay that offers not only a wide dynamic range but also a low detection limit.
Figure 3 illustrates a comprehensive laboratory testing algorithm for making the initial diagnosis and/or prognosis of HCV infection, as well as a follow-up testing regimefor patients receiving combo-therapy. After starting therapy, physicians monitor patients' HCV RNA levels to judge treatment efficacy, verify SVR, and to determine if the virus has been eradicated. Other treatment protocols require different testing regimens and are beyond the scope of this article.
Figure 3Combo-therapy Treatment Protocol
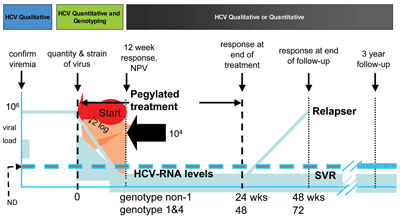 After diagnosis, patients receiving combo-therapy should be monitored to judge treatment efficacy. HCV RNA levels should fall if treatment is working.
After diagnosis, patients receiving combo-therapy should be monitored to judge treatment efficacy. HCV RNA levels should fall if treatment is working.
Better Treatments Ahead?
Untreated chronic HCV infection can lead to cirrhosis, hepatocellular carcinoma, and extra-hepatic disease. Until the general population can routinely be vaccinated against HCV infection, or individuals modify their high-risk behavior, infected patients should be offered therapeutic intervention. Interferon has been the mainstay therapeutic option, and novel regimens continue to be evaluated in clinical investigations.
The addition of ribavirin to pegylated interferon therapy clearly marks a significant advance in the treatment of chronic HCV. The introduction of the drugs boceprevir and telaprevir also represents a significant advance in treating genotype 1 chronic HCV infection. Additional work is still required to ensure that new and more effective therapies, including therapeutic options directed against HCV genotypes other than genotype 1, become available.
REFERENCES
- Centers for Disease Control and Prevention. Viral hepatitis. www.cdc.gov/hepatitis (Accessed April 2, 2012).
- Davis GL, Albright JE, Cook SE, et al. Projecting future complications of chronic hepatitis C in the United States. Liver Transpl 2003;9:331–8.
- Halliday J, Klenerman P, Barnes E. Vaccination for hepatitis C virus: closing in on an evasive target. Expert Rev Vaccines 2011;10:659–72.
- Simmonds P, Holmes EC, Cha T-A, et al. Classification of hepatitis C virus into six major genotypes and a series of subtype by phylogenetic analysis of the NS-5 region. J Gen Virol 1993;74:2391–9.
- Centers for Disease Control and Prevention. Hepatitis C information for health professionals. www.cdc.gov/hepatitis/HCV/ HCVfaq.htm (Accessed March 20, 2012).
- Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMRW 2012; 61:1–18
- Ge DL, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 2009;461:399–401.
- Alter MJ, Kuhnert WL, Finelli L. Guidelines for laboratory testing and result reporting of antibody to hepatitis C virus. MMWR Recomm Rep 2003;52:1–15.
- McHutchison J, Everson GT, Gordon SC, et al. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med 2009;360:1827–38.
- Poynard T, Marcellin P, Lee S, et al. Randomised trial of interferon alfa-2b and ribavirin for 48 weeks or for 24 weeks versus interferon alfa-2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. Lancet 1998;352:1426–32.
- Davis GL, Lau JY. Factors predictive of a beneficial response to therapy of hepatitis C. Hepatology 1997;26:122S–7S.
- Diestag JL, McHutchison JG. American Gastroenterological Association technical review on the management of hepatitis C. Gastroenterology 2006;130:231–64.

Peter Chou, PhD, is director of scientific affairs at Quest Diagnostics Nichols Institute, Chantilly, Va.
Email
Disclosure: Dr. Chou discloses that he has a financial interest in Quest Diagnostics (salary and stock).

Rick L. Pesano, MD, PhD, is medical director of infectious disease at Quest Diagnostics Nichols Institute, San Juan Capistrano, Calif.
Email
Disclosure: Dr. Pesano discloses that he has a financial interest in Quest Diagnostics (salary and stock).