In some patients with infection, a systemic response called sepsis occurs. The infection, usually bacterial, can originate in many body parts, such as the lungs, intestines, urinary tract, or skin. For some reason, signals from the bacteria cause an exaggerated response and even induce the patient's immune system to attack the body's organs and tissues.
Though it has been recognized since ancient times, researchers recently have learned a great deal about sepsis. We now know that it represents a model of the immune response to injury. Furthermore, many patients with sepsis probably are not immunocompetent but rather immunosuppressed. Although the exact cause of sepsis remains elusive, this new view has influenced the search for novel diagnostic markers as well as new approaches to therapy.
As knowledge about sepsis advances, laboratories will need to keep abreast of these new discoveries. This article provides an overview of the current thinking about the definition of sepsis and its diagnosis and treatment, with particular emphasis on the laboratory's role in recognizing patients with the condition.
Definition of Sepsis
A 1991 consensus conference defined sepsis as the combination of infection with two or more features of the systemic inflammatory response syndrome (SIRS): altered body temperature; elevated pulse rate; elevated respiratory rate; and abnormal white blood cell count (1). An update to that original definition, published in 2003 (2), expanded the criteria to include other signs and symptoms commonly seen in critical illness (Table 1, below). In addition, the update recommended making the diagnosis of sepsis even if documentation of infection was lacking, as long as it was strongly suspected. This shift in focus recognized that most features of sepsis are characteristic, regardless of the nature of the infection, and that evidence clearly implicates the response, rather than the inciting microorganism, as the problem.
Table 1
Definitions of Sepsis
Criteria for the Systemic Inflammatory Response Syndrome (SIRS)
Two or more of the following are required:
- Body temperature >38°C or <36°C
- Heart rate >90 beats/minute
- Respiratory rate >20 breaths/minute (or arterial pCO2 <32 mmHg, indicating hyperventilation)
- White blood cell count >12,000/mm3 or <4000/mm3 (or >10% immature forms)
Definitions
- Sepsis = Infection + SIRS
- Severe Sepsis = Sepsis + Evidence of Organ Dysfunction
Note: The 2001 update to the definitions stresses that documentation of infection may not be required for the diagnosis of sepsis if strong suspicion exists. Other criteria, such as altered mental status, edema, hyperglycemia in the absence of diabetes, elevated C-reactive protein, or elevated procalcitonin also are included.
Source: References 1 and 2
The original model for sepsis focused on the inflammatory response to endotoxin, a lipopolysaccharide (LPS) found in the cell walls of gram-negative bacteria. Endotoxin is an example of what is called a pathogen-associated molecular pattern (PAMP). Innate immune cells, such as macrophages, have developed surface receptors for PAMPs, named Toll-like receptors after a class of proteins first discovered in the fruit fly. In fact, the receptor for LPS was the first Toll-like receptor found in mammals. When engaged by a PAMP, these receptors stimulate macrophages to produce tumor necrosis factor (TNF), interleukin-1 (IL-1), and interleukin-6 (IL-6). These three pro-inflammatory cytokines produce SIRS, and for many years physicians believed that sepsis represented an overreaction on the part of the innate immune system to bacterial infection (Figure 1, below). However, neither traditional nor experimental anti-inflammatory treatment appeared to alter its course, casting some doubt on this theory.
Figure 1
Pro-inflammatory Phase of Sepsis
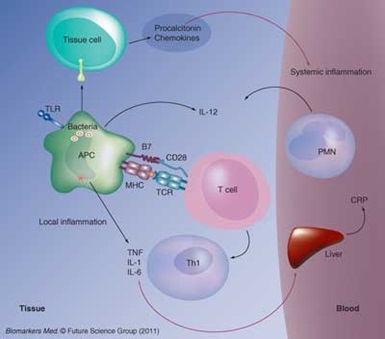
Sepsis begins when the innate immune system responds vigorously to the presence of bacteria. Toll-like receptors (TLR) cause the antigen-presenting cell (APC) to generate the pro-inflammatory cytokines (TNF, IL-1 and IL-6), and these produce systemic inflammation by activating circulating polymorphonuclear leukocytes (PMNs) and stimulating the liver to produce C-reactive protein (CRP). Procalcitonin and chemokines also are produced locally. The APC involves the adaptive immune system by presenting the bacterial antigen to the T cell receptor (TCR), using a Class II major histocompatibility complex (MHC) protein and co-stimulating by engaging CD28 with the B7 surface protein. In the presence of IL-12, this causes the T cell to become a pro-inflammatory Th1 cell.
Reproduced with permission from reference 5.
When sepsis does not resolve quickly, patients can develop widespread organ dysfunction, including lung, liver, and kidney injury. This condition is called severe sepsis, and the patient often dies. Even more deadly is septic shock, in which patients suffer cardiovascular collapse and often are unresponsive to fluid resuscitation and vasopressor therapy. The cause of the organ failure in severe sepsis is unknown, but it resembles multiple organ dysfunction syndrome (MODS) seen in patients who survive serious traumatic injury and do not have an infection. Autopsy studies by Hotchkiss and his colleagues demonstrated that patients who died of severe sepsis had significant depletion of T cells and B cells in the spleen (3). These observations were remarkably similar to findings seen in patients with organ dysfunction following trauma (4). Many investigators now consider both sepsis and post-traumatic MODS to be the same immunologic response to severe injury.
In this paradigm of sepsis, the innate immune system initially generates a pro-inflammatory state in response to PAMPs, or in the case of tissue injury, similar molecules derived from damaged host cells called damage-associated molecular patterns (DAMPs). Sometimes, this pro-inflammatory response produces a compensatory down-regulation of the immune system. Exactly why this occurs in some patients and not others is not known, but some other underlying factor or condition, or possibly genetic predisposition, may be involved. Similarly, why the immunosuppressed state is associated with organ dysfunction also is unclear.
Recognizing Sepsis
Despite the efforts of consensus conferences to better categorize the disease, patients with sepsis are heterogeneous and the condition is often difficult to recognize, especially in its early stages. The definition—SIRS plus evidence or suspicion of infection—may be too broad and include patients who are unlikely to experience significant morbidity or mortality. For instance, a young man in an emergency department with an infected foot and an elderly woman with pneumonia complicating her post-operative hospital stay could both be classified as having sepsis if they also have fever and an elevated white blood cell count. But they may not have the same risk of developing severe sepsis. Also, because the transition from sepsis to severe sepsis evolves over time in different ways in different patients, even the development of severe sepsis may escape recognition until it is too late. From the laboratory point of view, a biomarker capable of predicting which patients with sepsis are likely to develop severe sepsis would be a major asset.
Sepsis Biomarkers
The most commonly used biomarker of sepsis is blood lactate, most likely because elevated levels were inclusion criteria for early studies of intensive treatment of sepsis. The Surviving Sepsis Campaign, an international collaboration of medical organizations, still recommends obtaining a lactate level during patient monitoring. While lactate may be helpful in guiding resuscitation of severely ill patients, its role in early identification of patients at risk of developing severe sepsis is unclear. An elevated lactate level is associated with increased morbidity and mortality in sepsis, but using a relatively high cut-off, such as >4 mmol/L, may exclude many patients who probably need intervention. Studies have shown that even septic patients with relatively modest lactate elevations, >2 mmol/L, have increased mortality. On the other hand, such levels may also be seen in many patients without sepsis, complicating use of lactate in sepsis surveillance programs.
Many alternative biomarkers with greater specificity have been proposed to aid in diagnosing sepsis. These include: pro-inflammatory cytokines, such as IL-6; other proteins produced in response to infection and/or inflammation, such as C-reactive protein (CRP); markers of activated neutrophils, such as CD64; and, recently, novel biomarkers of the immunodeficient phase of sepsis (5). In experimental studies, some biomarkers have been able to successfully guide specific therapy. In clinical studies, however, few have sufficient specificity or sensitivity to be routinely employed in clinical practice, with the possible exception of procalcitonin (PCT).
Procalcitonin
PCT is the precursor of calcitonin, a hormone with no significant physiological role in humans, although it is capable of reducing plasma calcium levels when administered pharmacologically. In the early 1990s, investigators discovered elevated PCT in patients with invasive bacterial infection. Since then, numerous studies have investigated the diagnostic accuracy of PCT for sepsis, usually in comparison with CRP. Because PCT, unlike CRP, appeared to be synthesized only in response to bacterial infection and most infections associated with the development of severe sepsis are bacterial, many hoped that PCT would be a more sensitive and specific marker of sepsis. Despite many clinical studies, however, the role of PCT in the stratification of patients at risk for sepsis remains controversial.
Monitoring PCT levels in critically ill patients does appear to predict survival better than monitoring CRP. Furthermore, PCT levels correlate better with clinical scoring systems such as sepsis-related organ failure assessment (SOFA). But PCT's predictive power appears to be significant only later in the course of illness, and PCT levels vary early in infection. Consequently, although rising PCT levels may be helpful, elevated levels on admission to an intensive care unit probably are not. Recently, the results of a randomized trial testing whether knowledge of PCT levels resulted in more effective recognition and treatment of severe sepsis, did not support its use, and ironically, the patients in the PCT group had a longer hospital stay (6).
Immunosuppression Biomarkers
If indeed the transition from sepsis to severe sepsis involves a transition from an exaggerated pro-inflammatory state to an immunosuppressed state, novel biomarkers detecting immunosuppression may help recognize this transition early, perhaps even before the onset on organ dysfunction. Recently, many investigators have focused on the alterations in monocytes and T cells that appear to characterize the immunosuppressive phase of severe sepsis.
T cells recognize antigen in the context of major histocompatibility complex (MHC) class II proteins on the surface of antigen-presenting cells such as macrophages or dendritic cells. But, in addition to this signal, they also require engagement of a second, co-stimulatory molecule on their surface by a different protein, B7, on the antigen-presenting cell. T cells express two major types of ligands for B7: CD28 and CTLA-4. Engagement of CD28 by B7 stimulates the T cell; engagement of CTLA-4 by the same B7 molecule has the opposite effect (Figure 2, below). We now know that circulating monocytes from patients with severe sepsis have decreased amounts of MHC class II proteins on their surface and that circulating T cells have significantly increased CTLA-4. Whether these types of analyses, which are measured by flow cytometry, will help identify critically ill patients at risk of developing severe sepsis has yet to be determined.
Figure 2
Immunosuppressive Phase of Sepsis
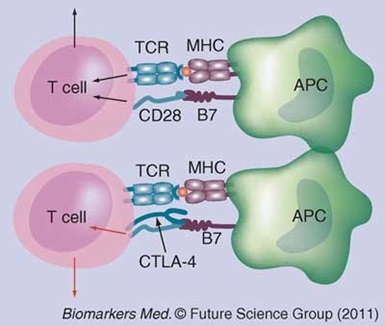
There is now significant evidence that patients with severe sepsis have defective adaptive immunity. T cells upregulate their expression of the “negative” co-stimulatory molecule CTLA-4. This surface protein competes with CD28 for binding to the costimulator B7 on the antigen-presenting cell (APC). Instead of activation, engagement of CTLA-4 reduces the T cell’s responsiveness and eventually causes it to undergo apoptosis (programmed cell death).
Reproduced with permission from reference 5.
Treatment of Sepsis
Once severe sepsis has been identified, within 6 hours physicians and nurses need to implement several key elements of the resuscitation bundle, a list developed by the Surviving Sepsis Campaign in collaboration with the Institute for Healthcare Improvement (7). These include obtaining blood cultures and administering broad-spectrum antibiotics and intravenous fluid to help support the patient's blood pressure. It is also important to differentiate the fluid resuscitation recommended by the Surviving Sepsis Campaign from early goal-directed therapy (EGDT). EGDT is an intensive strategy based on specific targets for central venous pressure and oxygen saturation, as well as arterial pressure. It requires special resources and appears to be underutilized, especially in settings such as the emergency department where the complexity of the protocol makes it difficult to implement and/or follow. Some also doubt whether the early reports of enhanced survival have held up, and there are currently three randomized trials underway to evaluate the efficacy of this approach (8).
Identifying the cause of the infection as quickly as possible and targeting antibiotic therapy appropriately are the next major objectives when managing severe sepsis. Because a significant percentage of patients with sepsis may have negative blood cultures, other potential sites of infection also need to be sampled. Molecular methods using either multiplexed, specific primers for the major microorganisms associated with sepsis or universal primers followed by species identification offer a potentially more sensitive way to identify the source (9).
Supportive measures directed at managing the organ dysfunction also are necessary. These include mechanical ventilation, monitoring urine output, and attention to proper nutrition.
In terms of treating the inflammatory response, conventional anti-inflammatory therapy, such as high-dose corticosteroids, has not been successful. In the last several years, small clinical trials utilizing low-dose corticosteroids have demonstrated a potential benefit in patients with refractory septic shock (10). Experimental studies using a mouse model of sepsis have shown that a modest down-regulation of the early pro-inflammatory phase of sepsis using low-dose corticosteroids improves survival and that pro-inflammatory markers such as IL-6 can help to identify subjects most likely to benefit from this treatment.
Disseminated intravascular coagulation (DIC) is extremely common in severe sepsis patients. Consumption of coagulation factors and platelets, coupled with inhibition of the fibrinolytic system, results in microvascular fibrin deposition. This compromises blood flow and is believed to contribute to the organ dysfunction characteristic of severe sepsis. Although the reason for the high prevalence of DIC in patients with sepsis is not well-understood, much attention has focused on the down-regulation of a natural inhibitor of the coagulation cascade, thrombomodulin, which is located on the vascular endothelial surface. In the absence of this regulatory protein, which binds thrombin and activates protein C, the procoagulant factors Va and VIIIa are not inhibited. This failure to impede coagulation led to the development of the only specific pharmacologic agent for treatment of sepsis approved by the U.S. Food and Drug Administration: recombinant human activated protein C (drotrecogin alfa, Xigris ® Eli Lilly). The drug's approval for patients with severe sepsis and a high risk of death was controversial, and its efficacy has been disputed. Recently, however, the manufacturer announced that it was withdrawing the drug from the market.
The recognition that severe sepsis likely represents a relatively immunosuppressed state suggests that restoring immunocompetency may be the optimal supportive strategy (Figure 3, below). A number of experimental studies aimed at neutralizing negative co-stimulatory molecules such as CTLA-4 or decreasing the production of anti-inflammatory cytokines such as IL-10 are underway (11). The potential success of such approaches is suggested by the discovery that mice lacking the gene for a negative co-stimulatory molecule called PD-1 have enhanced survival and reduced organ damage in the experimental model of sepsis.
Figure 3
The Two Phases of Sepsis
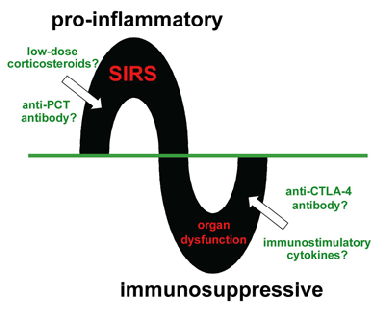
Sepsis may be divided into a pro-inflammatory phase characterized by the systemic inflammatory response syndrome (SIRS), followed by an immunosuppressive phase during which organ dysfunction develops.
Finally, PCT has also emerged as a potential target for intervention. Besides being a marker of sepsis, PCT may also contribute to reduced survival in patients with sepsis due to toxic pro-inflammatory effects on leukocytes and cytokine production. Investigators treated animals with experimental sepsis with antibody to PCT and were able to show marked improvement in survival. This effect was evident both after sepsis was established, as well as when immuno-neutralization was performed at the onset, indicating that early treatment may be effective (12).
Improving Sepsis Diagnosis and Treatment
No single marker of sepsis may be ideal today, but many are helpful in terms of at least identifying critically ill patients who need more careful monitoring. Future studies should incorporate novel biomarkers that target the immunoregulatory defects responsible for severe sepsis. Recent evidence hints that this new way of looking at sepsis may provide more effective approaches to treatment of patients with significant organ dysfunction. It makes sense that these should be paired with new approaches to recognizing severe sepsis, especially at an early stage.
Ultimately, the best way to recognize severe sepsis early is to increase awareness on the part of the nursing staff, and coordinate their observations with other pieces of information about the patient. Many healthcare organizations are starting to use computer programs to tie together the results of physiological monitoring, laboratory testing results, and clinical observations in order to identify patients at risk (13).
REFERENCES
- American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992;20:864–874.
- Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003;31:1250–1256.
- Hotchkiss RS, Tinsley KW, Swanson PE, et al. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol 2001;166:6952–2963.
- Choileain NN, Redmond HP. The immunological consequences of injury. Surgeon 2006; 4:23–31.
- Faix JD. Established and novel biomarkers of sepsis. Biomarkers Med 2011;5: 117–130.
- Jensen JU, et al. For the Procalcitonin and Survival Study (PASS) Study Group: Procalcitonin-guided interventions against infections to increase appropriate antibiotics and improve survival in the intensive care unit: A randomized trial. Crit Care Med 2011;39:2048–2058.
- Levy MM, Dellinger RP, Townsend SR, et al. The surviving sepsis campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med 2010;38: 367–374.
- Daniels R. Surviving the first hours in sepsis: Getting basics right (An intensivist's perspective). J Antimicrob Chemother 2011;66 Suppl 2:ii11–1123.
- Dark PM, Dean P, Warhurst G. Bench-to-bedside review: the promise of rapid infection diagnosis during sepsis using polymerase chain reaction-based pathogen detection. Crit Care 2009;13:217.
- Minneci PC, Deans KJ, Eichacker PQ, Natanson C. The effects of steroids during sepsis depend on dose and severity of illness: an updated meta-analysis. Clin Microbiol Infect 2009;15:308–318.
- Christaki E, Anyfanti P, Opal SM. Immunomodulatory therapy for sepsis: an update. Expert Rev Anti Infect Ther 2011;9:1013–1033.
- Becker KL, Snider R, Nylen ES. Procalcitonin in sepsis and systemic inflammation: a harmful biomarker and a therapeutic target. Brit J Pharmacol 2010;159:253–264.
- Rollins G. The State of Sepsis Care. Clinical Laboratory News 2011;37(3).

James Faix, MD, is director of clinical chemistry and immunology and clinical professor of pathology at Stanford University Medical Center. He is currently the chair of AACC's Divisions Management Group and a member of the Program Coordinating Committee.
Email
Disclosure: James Faix, MD, has received honorarium/expenses from BioMerieux, Inc.