Endorsement from an expert panel has put the Food and Drug Administration (FDA) on track to approve the first rapid, home HIV test, signaling a turning point in how the nation might handle the next phase of the 30-year long struggle with the disease. The unanimous recommendation from the FDA Blood Products Advisory Committee (BPAC) at its May meeting came as a surprise to some observers, given somewhat disappointing results from a clinical trial conducted by the test's manufacturer, OraSure Technologies.
The trial showed that the OraQuick test is less sensitive in the hands of untrained home users compared to the widely used professional version of the oral fluid-based test. But at the end of the day, BPAC heard impassioned testimony from a variety of HIV/AIDS community advocates and physicians in support of approving the test, and heeded FDA's own risk analysis, which predicted some 45,000 new HIV diagnoses that would otherwise go undetected in the first year of marketing the test to consumers.
Public health experts are watching closely to see what FDA does next. The agency does not have to follow the advice of its advisory committees, but usually does. "Any opportunity to increase the number of people who are aware of their HIV infection is potentially valuable from a public health standpoint," said Nicholas Moss, MD, director of clinical HIV prevention at the San Francisco Department of Public Health. "Some people are not comfortable testing for HIV in the settings that we routinely rely on in public health programs, and we might be able to reach them through home-based testing. That said, we always approach the rollout of new technologies with a lot of caution. If the product makes it to widespread use, we will be eager to see what the post-marketing data looks like in terms of false-negative and false-positive results."
A Bumpy Road toward Approval
OraSure is not the first company to approach FDA about an over-the-counter (OTC) HIV test. In fact, the discussion over home HIV testing has gone on for more than 20 years. FDA rejected requests for OTC HIV tests as early as 1989. In 1996, the agency approved two home-use blood specimen collection kits, but these required the user to mail a self-collected specimen for lab testing and wait for results over the phone or in the mail.
Meanwhile, since 2001 FDA has approved seven rapid HIV tests for professional use and granted CLIA waivers to four, including the OraQuick ADVANCE HIV 1/2—the test currently under consideration for the OTC market (See Box, below). CLIA waivers allow use of the tests in outreach efforts and other settings outside the lab, and OraSure has sold more than 25 million units in the professional market, including doctors offices, hospitals, and clinics.
Proposed OraQuick in-Home HIV Test Kit
The test now under consideration by FDA is nearly identical to the OraQuick ADVANCE HIV 1/2 professional-use kit. However, the test would be packaged in a laptop box that contains a pre- and post-test informational booklet, as well as step-by-step instructions on performing the test and interpreting results. The company plans to have trained telephone counselors available who speak English and Spanish. With over-the-counter approval from FDA, OraSure could sell the test in drug stores, convenience stores, supermarkets, and other retail locations.
The test kit contains the following items:
- Outer carton containing plastic molded laptop box
- Flipchart instruction Booklet attached to the plastic molded laptop box
- Test Device
- Developer Vial
- Pre-Test Informational Booklet (HIV, Testing & Me)
- Post-Test Informational Booklet (What Your Results Mean to You!)
- Pencil for writing down the read times
- Disposal Bag
- Information about contacting the OraQuick Answer Center
OraSure's talks with FDA about an OTC version of their test began in 2005, and in 2006, BPAC laid out guidance to the company on running a clinical trial that prospectively studied unobserved, home use of the test.
FDA's changing view toward home testing is due to improvements in technology, as well as an evolution in public health thinking, according to an agency brief released prior to BPAC's May meeting. Still, BPAC expressed strong concerns about the real-world performance of the test by lay users, and recommended an acceptable minimum performance for sensitivity and specificity as 95% at the lower bound of the 95% confidence interval (CI). The professional version of the test has a sensitivity and specificity of 99.3% (95% CI: 98.4–99.7%) and 99.8% (95% CI: 99.6–99.9%), respectively.
OraSure's final clinical trial for the home-use version enrolled 5,798 subjects from 17 high prevalence sites and three low prevalence sites across the country. OraSure gave subjects the test to take home and perform themselves, but also collected blood samples to compare to the results of the home-based testing. In this trial, the specificity of the test remained relatively high, 99.98% (95% CI: 99.90–100%), and above BPAC's recommended threshold.
However, sensitivity dropped in comparison to professional use of the kit to 92.98% (95% CI: 86.64–96.92%)—notably with 86.64% for the lower bound of the 95% CI. Sensitivity expresses the potential for false-negative results. OraSure was unable to determine the root cause for the one false-positive and eight false-negative test results in the trial, which were not associated with any specific demographic, according to a company spokesperson.
Despite this greater risk for false-negative results, the focus of the May BPAC meeting returned again and again to the high number of HIV-infected people who remain unaware of their status. "I still can't get past the quarter of a million people in the U.S. who have HIV and are not tested," said BPAC member Steven Pipe, MD. "If we make any dent in that, the benefits outweigh its risks." Pipe is an associate professor of pediatric hematology/oncology at the University of Michigan Health System in Ann Arbor, Mich.
|
Results from OraQuick In-Home HIV Test Clinical Trial
|
|
Criteria
|
Value |
95% CI |
| Sensitivity |
92.98% |
86.64–96.92% |
| Specificity |
99.98% |
99.90–100.0% |
| Accuracy |
99.84% |
99.69–99.93% |
| Positive-predictive value |
99.07% |
94.90–100.0% |
| Negative-predictive value |
99.85% |
99.71–99.94% |
|
Abbreviation: CI, confidence interval
Source: Food and Drug Administration
|
Balancing Risks and Benefits
In addition to the research presented by OraSure, FDA developed its own risk analysis model based on published data about HIV prevalence, transmission rates, OraSure's clinical trial, and other data. This analysis produced the estimate that the test could pick up 45,000 infections that otherwise would not be detected through existing testing modalities. The model predicts that 2.8 million people would use the test in the first year.
However, the FDA risk model also found that, based on the test's 92.98% sensitivity in clinical trials, the ratio of true positives to true negatives would be 13 to 1, with an estimated 3,800 false negatives. BPAC had recommended a ratio of 19 to 1. "There would be public-health benefits," said Richard Forshee, PhD, the associate director for research in the Office of Biostatistics and Epidemiology at FDA, who presented the analysis. "But an individual risk remains in the form of an increase in the number of false negatives."
The Centers for Disease Control and Prevention (CDC) presented data at the meeting that outlined the steep challenges the nation faces in controlling the epidemic. For example, of the approximately 1.2 million people in the country infected with HIV, only 80% know their status, less than half are retained in HIV care, and only 28% have a suppressed viral load (≤200 copies/mL).
An OraSure spokesperson emphasized that in their clinical trial, 96% of the newly identified HIV-positive subjects said they planned to follow-up with a doctor or clinic for treatment, and 41% reported never having tested before for HIV.
According to Tom Donohue, an HIV educator and founder of the advocacy organization, Who's Positive, the overall positive impact of a rapid OTC test would outweigh the risks. "I think this could revolutionize the way we think and act about HIV testing. This will be a new tool that will allow a whole new population of people who either find barriers to testing or have not been tested at all, to empower themselves to know their HIV status," he said. "I know there are some risks associated with the accuracy of the test, but on the other hand, if we want to become the generation that ends AIDS, I think we have to offer people as many options as possible."
Several members of BPAC expressed a similar sentiment. "We should not let the perfect be the enemy of the good, and this test is an additional option," said panel member Francisco Rentas, PhD, chief of blood services and director of education and training at Walter Reed Army Medical Center in Bethesda, Md.
Donohue emphasized that the test will appeal to a younger generation that demands quick answers and a more personalized, self-directed approach to their health. "For me, being a younger person living with HIV and talking to a lot of younger people, we are a technology-driven generation where people want new tools and new technology at their disposal. They want to know things right now. We're the generation that's growing up with Twitter, e-mail, and smart phones," he said. "I think for us, there is an expectation for newer, faster technology, and this test provides a rapid response to a question that people want to know."
Donohue also contended that barriers to HIV testing still remain for many people. For example, he has spoken to those in rural areas who do not want to take the risk of being identified by someone they know if they go to a clinic for testing. "Even though an older generation may be used to going to a clinic and getting that very valuable pre- and post-test counseling, not everyone wants that," he said. "Of course a younger generation would gravitate to this type of a test. They don't have to talk to a counselor if they don't want to, and they don't have to face someone in their own community to talk about their sexual behaviors and take all the risks that go along with that."
A central question for public health authorities will be how to make sure that people who learn of their HIV positive status at home get connected with needed healthcare resources, noted Moss. "With any home-based testing technology, we need to be really clear to the users that a positive test needs confirmation, and then if they truly are positive, they can be engaged in HIV care though that process," he said. "We need to make sure that people are able, comfortable, empowered to act on the positive test result that they've received from testing themselves."
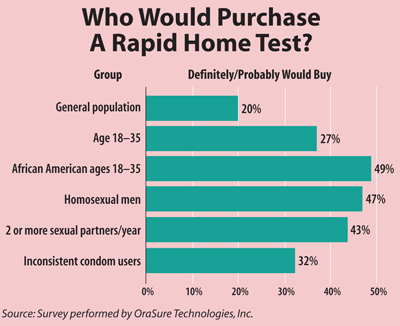
Moss said he expects there to be demand for an OTC test among both high- and low-risk groups (See Box, below). "I think there are high-risk people who appreciate the importance of getting tested for HIV often and will really appreciate being able to take the test at home," he said. Moss also expressed interest in finding ways for public health departments such as his own to incorporate OTC testing into their programs.
At the meeting, BPAC members also focused on more practical issues, such as what kind of labeling an OTC kit would need, and how concepts like the window period before seroconversion should be explained. Arleen Pinkos, an expert scientific reviewer for point-of-care testing at FDA, warned BPAC that there are "always surprises" with rolling out OTC devices. "Despite instructions, users always take shortcuts, and few read the labeling," she said. "Many people do not understand false-positive and false-negative results—they think, ‘FDA wouldn't clear a bad test'.''
Lab Algorithm Changing Soon
At the same time that FDA is considering putting HIV testing in the hands of consumers, laboratories should expect a new CDC-recommended algorithm for lab testing by the end of the year. The new algorithm drops traditional Western blot testing and calls for initial screening with a fourth-generation immunoassay. Positive results would be followed by an HIV-1/HIV-2 differentiation immunoassay, and a negative result at this step would lead to nucleic acid amplification testing (NAAT). According to CDC, the new algorithm will offer several benefits, including detecting both acute and long-standing infection, differentiating HIV-1 from HIV-2, providing more timely results, and eliminating indeterminate and inconclusive results.
CDC has been working on the new algorithm for several years, and is now completing the final recommendation. "In discussing it with people here at CDC as well as other laboratory directors, we feel that at the current time we have a sufficient amount of validation data for the new algorithm," said Bernard Branson, MD, associate director of laboratory diagnostics in the division of HIV AIDS prevention in CDC's National Center for Viral Hepatitis, HIV, STD and TB Prevention. "Currently, we are looking at some of the cost-effectiveness data, which is one of the ingredients CDC uses when it is putting together recommendations. We are in the process of drafting those recommendations now, but obviously will continue to monitor data as it accumulates."
One of the most influential studies examining the new algorithm came from the New York State Department of Health, according to Branson. This study looked at 38,257 specimens and found that, compared to the old algorithm, the new algorithm was more sensitive for detecting HIV-1 infection, provided a greater number of definitive HIV results, and detected HIV-2 more efficiently (J Clin Virol. 2011; 52 Suppl 1:S35–40).
Research is ongoing on how fourth generation assays can detect HIV earlier. These assays detect both HIV-1 and HIV-2 antibodies as well as HIV p24 antigen, which spikes before seroconversion and tightens the test's window period. Currently, both Abbott and Bio-Rad have FDA-approved tests.
Moss is participating in a CDC-funded study called STOP that is evaluating fourth generation assays, comparing them with pooled RNA testing to detect acute infection. "The question is, we're detecting people a few weeks earlier with this technology. Is that going to have a significant public health benefit in terms of reducing transmission and getting people into care earlier versus the existing testing technology?" Moss said. "We're trying to answer some of those questions by working with the CDC, along with other sites in New York and North Carolina."