With most attention today focused on drugs-of-abuse, toxic alcohol poisonings are often overlooked. Yet ethylene glycol, methanol, and isopropanol, which are found in products that are inexpensive and readily obtainable, can cause significant harm to individuals who ingest them (1). Toxic alcohol poisonings occur in accidental ingestions, self-harm attempts, or when isopropanol is used as a substitute for ethanol in drinks. Of the three compounds, ethylene glycol and methanol are particularly dangerous, mainly due to metabolites formed in the body that can cause severe organ damage.
Laboratory diagnosis of toxic alcohol ingestions presents several practical challenges (1,2). While most clinical laboratories can quickly and accurately quantitate plasma or serum ethanol levels using enzymatic assays on common chemistry analyzers, rapid assays for other alcohols have not been commercially available or have lacked specificity. The gold standard assay for determining toxic alcohol concentrations in plasma or serum is gas chromatography (GC), a labor-intensive technique not available in most clinical settings.
Many hospitals typically send out specimens for GC analysis, thereby precluding the 2–4 hour turnaround time for toxic alcohol analysis recommended by a National Academy of Clinical Biochemistry consensus panel (3). Clinical diagnosis can be challenging given that patients who have ingested toxic alcohols often present with non-specific signs and symptoms. They also come to the emergency department in an altered state of consciousness, or are semi-conscious, or even unconscious and unable to provide a reliable history (1,3).
Because toxic alcohol poisonings can be fatal for patients, laboratories need to be prepared to meet the challenges of providing accurate diagnostic test results. This article will review the characteristics of toxic alcohols and focus on recent advances in their laboratory analysis.
Characteristics of the Toxic Alcohols
Ethylene glycol is a sweet-tasting compound found in most automobile antifreezes (1). The pleasant taste of ethylene glycol contributes to accidental poisoning in children as well as pets. Individuals also sometimes ingest it deliberately in self-harm attempts. The body metabolizes ethylene glycol to glycolic acid and oxalic acid. The latter metabolite has the potential to cause severe renal injury by precipitating with calcium in the renal tubules.
Windshield fluid, a variety of cleaning solvents, canned cooking fuel, and even some liquid fuels for model railroad sets contain methanol (1). Although it has a relatively sweet odor, ingesting methanol leads to a burning, unpleasant taste, which likely explains why it is a less common cause of poisoning than ethylene glycol. The body metabolizes methanol to formic acid, a toxic compound that can cause blindness as a result of permanent injury to the optic nerve.
Rubbing alcohol is the most common source of isopropanol. People sometimes mistakenly think that isopropanol is a safe substitute for ethanol. Others drink it in self-harm attempts, including in hospital settings, such as a delirious or suicidal patient who gains access to rubbing alcohol bottles intended for medical purposes. Ingesting isopropanol is generally less serious clinically than ethylene glycol or methanol, as it is primarily metabolized to acetone. Individuals commonly experience gastrointestinal symptoms, including vomiting, that effectively limit the amount of the compound absorbed systemically.
In addition to specific organ damage caused by the metabolites of ethylene glycol and methanol, all the toxic alcohols also are capable of producing substantial respiratory and central nervous system (CNS) depression. These effects can be life-threatening, especially with large overdoses. In fact, toxic alcohol ingestion can be fatal due to respiratory failure, brain injury, or accidental trauma that occurs during intoxication. Furthermore, the fatality risk increases when individuals also ingest other CNS or respiratory depressants like ethanol, benzodiazepines, or opiates.
An additional compound sometimes classified as a toxic alcohol is propylene glycol (1). Although chemically similar to ethylene glycol and also used in some brands of automobile antifreeze, propylene glycol is generally much less toxic than ethylene glycol. A variety of products, including cosmetics, ointments, and some activated charcoal preparations, contain propylene glycol. It also is used as a diluent for intravenous preparations of poorly water-soluble drugs, including lorazepam, diazepam, and etomidate. Researchers have described toxicity resulting from overdoses of propylene glycol-containing antifreeze and repeated intravenous administrations of medications containing propylene glycol as the diluent. In particular, patients under extended sedation with lorazepam, such as intubated patients on mechanical ventilation, can suffer toxicity.
Table 1 summarizes the characteristics of the toxic alcohols.
|
Table 1
Common Sources, Clinical Signs, and Analytical Methods for Alcohols
|
|
Alcohol
|
Common Sources
|
Osmolal Gap
|
Anion Gap
|
Analytical Methods
|
| ethanol |
alcoholic beverages |
present
|
possible
|
enzymatic assay, GC |
| ethylene glycol |
automobile antifreeze |
present
|
present
|
GC, GC/MS,
enzymatic assay |
| isopropanol |
rubbing alcohol |
present
|
absent
|
GC |
| methanol |
windshield fluid, cooking fuels |
present
|
present
|
GC |
| propylene glycol |
activated charcoal, intravenous medications |
present
|
possible
|
GC, GC/MS |
| Abbreviations: GC, gas chromatography; GC/MS, gas chromatography/mass spectrometry |
Treatment of Toxic Alcohol Ingestion
Patients who have ingested ethylene glycol or methanol receive antidotal treatment with fomepizole (4-methylpyrazole) or ethanol (4,5). Both compounds prevent the first, rate-limiting enzymatic reaction catalyzed by alcohol dehydrogenase. Fomepizole is a competitive antagonist of alcohol dehydrogenase, while ethanol is a substrate for the enzyme that also competes with ethylene glycol and methanol. Fomepizole and ethanol act as antidotes by preventing the enzymatic conversions of ethylene glycol and methanol to toxic metabolites, allowing the kidneys to clear the toxic alcohols.
Treatment with fomepizole has steadily increased in the U.S. because it is easier to administer than intravenous ethanol. Furthermore, ethanol has the potential to further contribute to the patient’s CNS and respiratory depression in cases of ethylene glycol and methanol ingestion. However, the high cost of fomepizole, more than $1,000 per dose, presents another challenge, especially for hospitals that rarely encounter ethylene glycol or methanol ingestions. Some networks of hospitals now share the drug, alleviating the need for each to stock it individually.
Antidotal therapy with fomepizole or ethanol, however, may not be sufficient in patients who present more than several hours after ingestion or for those who have ingested very large amounts. Laboratory findings of patients with late-presenting ingestions include metabolic acidosis, increased anion gap, and glycolic acid (1,6). In either of these situations, patients should undergo renal dialysis to clear both parent drug and their metabolites (4,5). Although quite effective in managing toxic alcohol ingestion, renal dialysis is not a simple procedure and requires specialized personnel.
Laboratory Diagnosis of Toxic Alcohol Ingestion
While some patients have observable oxalate crystals, especially 8 hours or more after ingestion of ethylene glycol, examining urine for oxalate crystals should not be used a screening test for ingestion due to low sensitivity and specificity. The preferred method to detect and quantitate ethylene glycol, isopropanol, and methanol is GC with flame ionization coupled with a technique called head space analysis that eliminates time-consuming liquid extractions (1,2). Laboratories also can measure acetone, a metabolite of isopropanol, and glycolic acid, a metabolite of ethylene glycol, by GC. GC has high specificity for the toxic alcohols, with limits of detection of ≤ 10 mg/dL. Due to their higher volatility, ethanol, isopropanol, and methanol analysis requires a different procedure than ethylene glycol, which some laboratories analyze by gas chromatography/mass spectrometry (GC/MS).
The downside of GC and GC/MS is that both are labor-intensive and not automated. In hospitals that encounter toxic alcohol poisonings infrequently, clinical laboratory managers may find it difficult to justify performing GC in-house, especially when adding staff already is challenging. An additional consideration is that many toxic alcohol ingestions occur at night when laboratory staffing is often at minimum levels. Consequently, diagnosis of toxic alcohol ingestion often depends on indirect laboratory findings, such as osmolal gap, acidosis, and anion gap.
An elevated osmolal gap is an early sign of toxic alcohol as well as propylene glycol ingestion, leading to the widespread use of this measurement as a screening test. Laboratories determine osmolality by measuring the freezing point depression of the patient’s serum and estimating the osmolality contribution of the endogenous major contributors—sodium, blood urea nitrogen (BUN), and glucose—from an equation. By definition, the osmolal gap is the measured osmolality minus the estimated osmolality. Some equations also take into account the presence of serum ethanol.
Laboratorians should be aware that there is considerable debate about the use of osmolal gap to diagnose toxic alcohol ingestions, as well as a plethora of proposed empiric equations for estimating the contribution of sodium, BUN, glucose, and ethanol to serum osmolality (7,8). Table 2 summarizes the laboratory and calculated parameters used in the diagnosis of toxic alcohol ingestions.
Click for Table 2
An elevated osmolal gap, defined as >10–15, suggests the presence of additional osmotically active substances in blood. But differential diagnosis of an elevated osmolal gap is broad and also includes alcoholic ketoacidosis, which is seen in some patients following ethanol binges, diabetic ketoacidosis, mannitol infusion, renal failure, and shock. Alcoholic ketoacidosis also produces a substantial osmolal gap even in the absence of detectable plasma ethanol due to the formation of glycerol, acetone, and the acetone metabolites acetol and 1,2-propanediol in patients.
How Good is Osmolal Gap?
Despite the widespread clinical use of osmolal gap for the diagnosis of toxic alcohol ingestion, few studies have rigorously evaluated the diagnostic performance of this calculated parameter as a screening test. In one study, Lynd, et al., looked at retrospective data over a 6-year period from two tertiary care hospitals in Canada and found that osmolal gap was highly sensitive for toxic alcohol ingestion at a cutoff of 10, with sensitivities approaching 1.0 for identifying toxic alcohol ingestions that required antidotal or renal dialysis therapy (9). The area under the curve (AUC) for receiver-operator characteristic (ROC) curve analysis range was 0.736–0.785, depending on the osmolality formula used. The researchers concluded that the main limitation of the measurement was lack of specificity, resulting in a positive predictive value of <0.5, even for osmolal gaps up to 30.
Another group, Krasowski, et al., recently analyzed nearly 15 years of retrospective data from a U.S. academic medical center to ascertain the causes of elevated osmolal gaps (10). In this study, the hospital laboratory offered a testing panel with plasma sodium, BUN, glucose, osmolality, and ethanol. The laboratory used the tests to calculate osmolal gap and as part of the decision process for pursuing GC analysis of either a panel of methanol, isopropanol, ethanol, and acetone or a panel of ethylene and propylene glycol. The retrospective study revealed that recent heavy ethanol ingestion was the single most common cause of an osmolal gap >14, with many of these cases having clinical histories and laboratory findings compatible with alcoholic ketoacidosis. The second most common cause was ingestion of toxic alcohols of any type. Other causes of elevated osmolal gap included diabetic ketoacidosis, renal failure, shock, and recent mannitol infusion (Figure 1).
Figure 1
Causes of Elevated Osmolal Gaps
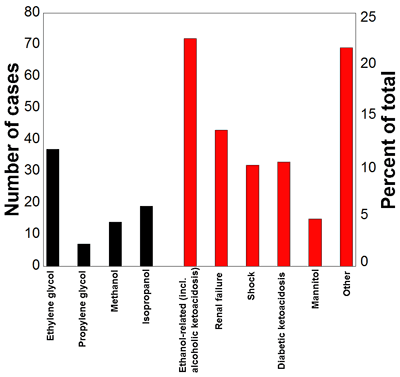 This graph shows the causes of elevated osmolal gap from a retrospective study. The black bars indicate number of patients with toxic alcohol ingestion as the likely primary cause of the elevated osmolal gap. The red bars represent suspected primary causes of elevated osmolal gap in the absence of toxic alcohol ingestions.
Source: Reproduced from reference 10 (BioMed Central publisher).
This graph shows the causes of elevated osmolal gap from a retrospective study. The black bars indicate number of patients with toxic alcohol ingestion as the likely primary cause of the elevated osmolal gap. The red bars represent suspected primary causes of elevated osmolal gap in the absence of toxic alcohol ingestions.
Source: Reproduced from reference 10 (BioMed Central publisher).
Many of the highest osmolal gaps occurred in patients ingesting toxic alcohols. For the patients who had elevated osmolal gaps resulting from some other cause, only 12% had an osmolal gap >30 on the initial laboratory work-up, 3% had >50, and none had an osmolal gap >100. In contrast, for patients who had a positive analysis for toxic alcohols by GC, 49% had an osmolal gap >30 on the initial laboratory work-up and 20% had an osmolal gap >50.
Enzymatic Assay for Ethylene Glycol
Until recently, rapid and specific assays for alcohols other than ethanol were unavailable. Although colorimetric and enzymatic assays for ethylene glycol have been used in veterinary settings, lack of specificity for human use has been a problem. In particular, the assays produce false positives in samples containing propylene glycol and other related compounds, as well as 2,3-butanediol found in some chronic alcoholics. To meet the clinical need for a rapid and specific ethylene glycol assay, a research team recently developed an enzymatic assay based on a commercially available glycerol dehydrogenase assay (11).
The veterinary assay, marketed by Catachem, accurately quantitates ethylene glycol in serum or plasma samples that do not contain chemically similar compounds such as propylene glycol. While the assay fulfills the needs of veterinary settings in which animals that have ingested ethylene glycol would be unlikely to have interfering compounds in their blood, human samples may contain 2,3-butanediol from chronic ethanol use or propylene glycol from prior administration of activated charcoal or intravenous medications. The researchers successfully modified the parameters for the assay so that it would run on the Hitachi 917 and Olympus AU400 automated analyzers and achieved a specific method unaffected by interference from propylene glycol, 2,3-butanediol, or similar compounds. This finding holds promise for clinical laboratories interested in rapid triage of possible toxic alcohol ingestions.
Overcoming the Diagnostic Challenges
As long as toxic alcohols are available in cheap and easily obtainable products, emergency departments will likely continue to see patients who have ingested these compounds. Except for a small number of hospitals that have rapid access to GC or GC/MS analysis, clinicians are forced to diagnose toxic alcohol ingestions based on clinical history and indirect signs. While some laboratories use osmolal gap as a screening test due to its high sensitivity for detecting toxic alcohol ingestions, clinicians need to be aware that this test has poor specificity. Patients may also have laboratory findings of metabolic acidosis and anion gap, but these appear much later and their absence should not be used to rule-out ingestions.
The recent study by Juenke and colleagues (11) suggesting that a rapid and specific ethylene glycol enzymatic assay has clinical utility for human specimens is an exciting development for clinical laboratories. Even for hospitals that see few ethylene glycol ingestions, the availability of a rapid ethylene glycol assay may prove attractive given the expense of fomepizole and the invasiveness of dialysis therapy. Clearly, the benefits of quickly ruling out ethylene glycol ingestion and focusing on other causes of altered mental status, especially in emergency departments that see large numbers of patients, is substantial.
However, clinical laboratories still need a rapid and specific clinical assay for methanol. Even though methanol ingestion is less common than ethylene glycol, a missed diagnosis could lead to patient harm. The ability to rapidly rule-out both ethylene glycol and methanol would be a further advance.
REFERENCES
- Kraut JA, Kurtz I. Toxic alcohol ingestions: clinical features, diagnosis, and management. Clin J Am Soc Nephrol 2008;3:208–225.
- Jialal I, Devaraj S. Laboratory diagnosis of ethylene glycol poisoning: the cup is half full? Am J Clin Pathol 2011;136:165–166.
- Wu AH, McKay C, Broussard LA, Hoffman RS, et al. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: recommendations for the use of laboratory tests to support poisoned patients who present to the emergency department. Clin Chem 2003;49:357–379.
- Barceloux DG, Krenzelok EP, Olson K, Watson W. American Academy of Clinical Toxicology practice guidelines on the treatment of ethylene glycol poisoning. Ad Hoc Committee. J Toxicol Clin Toxicol 1999;37:537–560.
- Barceloux DG, Bond GR, Krenzelok EP, Cooper H, et al. American Academy of Clinical Toxicology practice guidelines on the treatment of methanol poisoning. Ad Hoc Committee. J Toxicol Clin Toxicol 2002;40:415–446.
- Mycyk MB, Aks Se. A visual schematic for clarifying the temporal relationship between the anion and osmol gaps in toxic alcohol poisoning. Am J Emerg Med 2003;21:333–335.
- Khajuira A, Krahn J. Osmolality revisited —deriving and validating the best formula for calculated osmolality. Clin Biochem 2005;38:514–519.
- Purssel RA, Pudek M, Brubacher J, Abu-Laban RB. Derivation and validation of a formula to calculate the contribution of ethanol to the osmolal gap. Ann Emerg Med 2001;38:653–659.
- Lynd LD, Richardson KJ, Purssell RA, Abu-Laban RB, et al. An evaluation of the osmole gap as a screening test for toxic alcohol poisoning. BMC Emerg Med 2008;8:5.
- Krasowski MD, Wilcoxon RM, Miron J. A retrospective analysis of glycol and toxic alcohol ingestion: utility of anion and osmolal gaps. BMC Clin Pathol 2011;in press.
- Juenke JM, Hardy L, McMillin GA, Horowitz GL. Rapid and specific quantitation of ethylene glycol levels: adaptation of a commercial enzymatic assay to automated chemistry analyzers. Am J Clin Pathol 2011;136:318–324.

Matthew D. Krasowski, MD, PhD, is a clinical associate professor, medical director of the Clinical Chemistry and Point-of-Service Laboratories, and vice-chair for Clinical Affairs in the Department of Pathology at the University of Iowa Hospitals and Clinics in Iowa City. Email: [email protected]