
The largest show of lab products and services in the world opens today with greater than 700 exhibitors, once again breaking records, this year in Los Angeles. But the 2012 AACC Clinical Lab Expo is more than just an exciting opportunity for laboratorians from around the world to discover the latest technologies and trends. The robust show reflects the resiliency of the diagnostics industry during an extended, world-wide period of economic uncertainty.
Market reports show that the in vitro diagnostics (IVD) market has grown steadily despite a global financial crisis over the past 5 years. In fact, according to the healthcare market research and consulting firm Enterprise Analysis Corporation (EAC), the IVD market finally crossed the $50 billion mark in 2011 and is headed for nearly $70 billion by 2016. Some segments, like molecular diagnostics, are growing rapidly; yet traditional areas in clinical chemistry are holding their own. At the same time, labs and IVD companies have tough choices ahead, as lower reimbursement and other constraints brought on by the recession will remain for years to come, according to experts.
A Snapshot of Global Growth
According to EAC projections, the global IVD market will push ahead at a compound annual growth rate of approximately 6.3% through 2016, climbing up to $68.8 billion. As in recent years, emerging markets like Asia Pacific and Latin America will experience more rapid growth than North America or Europe (See Box, below).
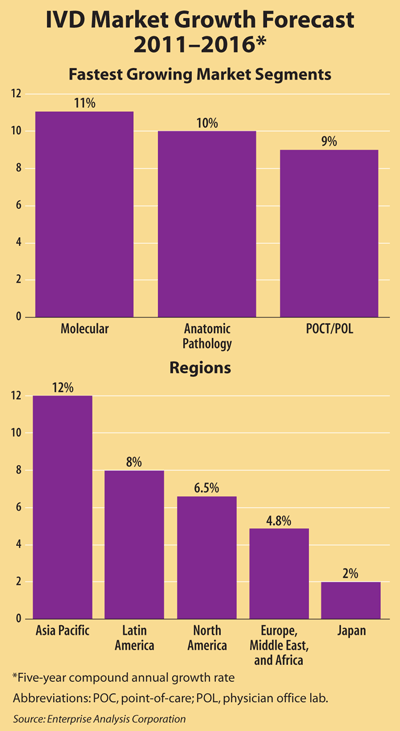
Due in part to faster adoption of new technologies in North America, EAC projects growth to be higher here than in Europe, at 6.5% compared to 4.8%. Even though many tests achieve regulatory approval more quickly in Europe than in the U.S., North American growth is comparatively stronger because of more intense strain on reimbursement in Europe, explained Gerard Conti, a vice president at EAC. "European markets have more control over reimbursement, and every government is trying very hard to control costs right now," Conti said. In Germany, for example, reimbursement is even lower than in France, Spain, and other European countries, even though it is the region's largest economy.
Surging growth in the BRIC countries—Brazil, Russia, India, and China—will keep growth in Asia Pacific and Latin America near 12% and 8%, respectively. "It's possible for growth in Asia Pacific to be even stronger," Conti said. "However, we prefer to be a little more conservative, since we don't know if current growth rates can stay so high through the next five years."
Robust POCT Segment Defies Cost Pressures
Despite shrinking hospital budgets, a recent EAC survey showed that U.S hospitals are still expanding point-of-care testing (POCT), even though POCT is often significantly more expensive than central lab testing. Taken together, POCT and physician office lab testing are projected to grow at about 9%, not a huge number but significantly higher than the 5-6% growth in traditional central lab testing.
EAC surveyed hospitals across the U.S. and found a continued trend of POCT adoption, explained Mark Hughes, vice president and a senior consultant at EAC. "In our survey, we found continued penetration of point-of-care testing products like the Abbott iSTAT, and more use of such platforms across disciplines—from blood gas to cardiac markers, chemistry, and coagulation," Hughes said. "Our data indicate that hospitals are still willing to find a way to purchase these more expensive point-of-care tests, and clearly they see value and benefit in point-of-care testing that makes it worth paying a premium price."
In the POCT area, several companies have advanced multiplexing platforms in the pipeline, some of which are designed to perform a broad range of immunoassays, and even molecular tests. Despite several companies aiming to launch these advanced POCT platforms soon, it will likely be a while before they actually hit the market, Hughes said. "Although some of these companies have been promising these new platforms for years now, I believe they really will be coming to the market. It just takes longer than the companies expect," he said. "I do think we'll see some real point-of-care molecular testing within the next five years. There are a lot of promising technologies in the pipeline."
Shifting Costs Around the Lab
Gauging the market from a lab perspective also shows growth, although it trails behind the numbers for the IVD manufacturer side, explained Winny Tan, PhD, an industry analyst with market research firm Frost and Sullivan. Tan projected a growth rate for the clinical lab market of 5.7% for the next 5 years. "Despite growth drivers such as an aging population, we see falling reimbursement and regulatory issues pushing back," Tan said. "Even with the potential for greater access to care through healthcare insurance reforms, and a greater number of patients seeking care, lower reimbursement is likely to overpass these trends and keep market growth in check."
Especially in hospitals, cost shifting acts as a buffer that restricts the impact on the lab from potential positives like efforts to increase access to care, Tan explained. "Even changes in physician reimbursement are going to impact the lab because of cost shifting, especially since hospital labs are usually seen as a cost center," she said. "So even an improvement in access to care or reimbursement in one area can be erased by another because these systems are interconnected."
Both for labs and diagnostic companies marketing tests, lower reimbursement across the board means the pressure is on to demonstrate the value of lab services, Tan said. "In the big picture of paying for healthcare, in vitro diagnostics are currently just not valuated as appropriately as drugs or professional services," she said. "Labs nowadays have the extra onus of not only showing clinical value to health plan providers, but creating economic efficiencies. So, if you reimburse this test, how much do you save in unnecessary treatments and other costs down the line?"
Even in the face of these challenges, some labs are managing to turn from a cost center into a profit center through outreach and other programs, Tan noted. But these are few and far between. For most hospital labs, efficiency is a more realistic goal than expansion. "It comes down to the fact that running a lab is really expensive," Tan said. "Some labs are building out with a greater footprint, which would be considered pretty aggressive. I think many labs can lean their operations, which may be a more feasible approach than building out and filling up with a lot of capital investment. There is just so much labs can do with efficiency, automation, new technology, and leveraging information technology as well."
Molecular Dx: Room to Grow
Even though molecular diagnostics remains a major growth driver for IVD manufacturers, so far the majority of labs continue to send out these tests, making the molecular lab market more concentrated. Based on Frost and Sullivan research, only about 800 laboratories in 2011 performed significant volumes of molecular testing in the U.S., Tan said. That is compared to the nation's 8,000 hospitals, and between 5,000 and 6,000 private commercial labs. This means molecular diagnostics can be a boon for labs that have the expertise—and the volumes—to be successful.
"Clinical chemistry and microbiology tests still make up at least 50% of the testing volume in the U.S.," Tan commented. "Although molecular volume is certainly growing, driven especially by infectious disease applications, it's not easy for labs to integrate these technologies. They have to ramp up the skill set of employees, and be sure they can reach enough test volume to warrant the investment into capital equipment."