In July 2023, we changed our name from AACC (short for the American Association for Clinical Chemistry) to the Association for Diagnostics & Laboratory Medicine (ADLM). The following page links to resources that were written prior to the rebranding and/or mentions events that took place prior to the rebranding and that contain mentions of the association’s old name.
Laboratory test results are a critical component of patient care. These values help physicians diagnose disease, are critical to developing clinical guidelines that direct treatment options, and are instrumental in ongoing efforts to improve and measure the quality of patient care. The Association for Diagnostics & Laboratory Medicine (formerly AACC) is working to improve clinical testing by resolving the lack of harmonization, or uniformity, among test results.
Most tests report a numeric value for healthcare providers to interpret. The range of numbers reported for a test for a certain condition may vary depending on the method used. Although labs generate test results that are correct within the context of a given method, this lack of harmonized or uniform results makes it difficult for healthcare providers or patients to compare results over time or across labs. Ensuring that labs report the same range of numeric values for a test (e.g., cholesterol), regardless of method or device, helps reduce medical errors, lower healthcare costs, and increase patients' involvement in their care decisions.
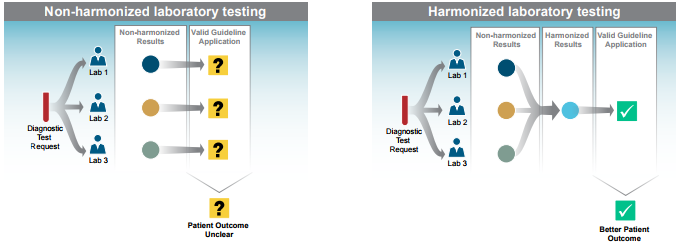
Harmonization improves patient outcomes by ensuring that tests provide the same results regardless of their manufacturer or the lab performing them.
Securing Congressional Funding for Harmonization
For several years, the Association for Diagnostics & Laboratory Medicine (ADLM) has spearheaded a collaborative initiative involving partners in the healthcare community to gain congressional support for harmonization. This initiative led Congress to include report language in its 2015 spending bill that directed the Centers for Disease Control and Prevention (CDC) to work with the private sector to harmonize clinical laboratory results. Subsequent ADLM-led efforts have led Congress to allocate $2 million in funding for the initiative every fiscal year since 2018. This funding has given CDC the resources to advance harmonization for select hormone tests, which will improve diagnosis and treatment for diseases such as hypothyroidism, chronic kidney disease, and osteoporosis. ADLM is working with its partners to secure additional funding for the upcoming fiscal year.
 ADLM’s lab experts Drs. David Carpentieri, Robert Fitzgerald, and Brett Holmquist visit Capitol Hill to discuss the need for lab test harmonization with Rep. Kevin Yoder (R-KS).
ADLM’s lab experts Drs. David Carpentieri, Robert Fitzgerald, and Brett Holmquist visit Capitol Hill to discuss the need for lab test harmonization with Rep. Kevin Yoder (R-KS).
 Together with CDC’s Dr. James Pirkle, ADLM experts Drs. Darius Paduch, Stephen Master, and David Koch hold a Congressional briefing on why harmonization is vital to quality patient care.
Together with CDC’s Dr. James Pirkle, ADLM experts Drs. Darius Paduch, Stephen Master, and David Koch hold a Congressional briefing on why harmonization is vital to quality patient care.
ADLM Position Statement on Harmonization
In this position statement, ADLM provides guidance on how laboratory organizations, clinical societies, regulatory agencies, the in vitro diagnostic industry, and other stakeholders can contribute to harmonization efforts.
Additional Resources
Policy Documents
Letters to Congress in Support of Harmonization
Congressional Briefing
Other Organizations
ADLM's Harmonization Partners
ADLM would like to thank the following organizations for their support of the association's efforts to secure congressional funding for harmonization:
- American Medical Technologists
- American Society for Clinical Laboratory Science
- American Society for Clinical Pathology
- American Society for Microbiology
- American Society of Hematology
- American Thyroid Association
- American Urological Association
- ARUP Laboratories
- Association for Molecular Pathology
- Association of Maternal & Child Health Programs
- Association of Public Health Laboratories
- Association of Schools and Programs of Public Health
- Big Cities Health Coalition
- COLA, Inc.
- College of American Pathologists
- Endocrine Society
- Infectious Diseases Society of America
- Laboratory Corporation of America Holdings
- National Association of Pediatric Nurse Practitioners
- National Network of Public Health Institutes
- PCOS Challenge: The National Polycystic Ovary Syndrome Association
- Pediatric Endocrine Society
- Quest Diagnostics
- Roche Diagnostic Corporation
- Siemens Healthcare Diagnostics
- Society for Reproductive Investigation