
Cardiac troponin (cTn) is the biomarker of choice for detecting myocardial necrosis and assessing acute ischemic changes observed with acute coronary syndrome (ACS) and myocardial infarction (MI, type 1). Current techniques detect two discrete myocardial-specific forms of cTn: cTnI and cTnT. Following myocardial necrosis—cell death—myocardial cells release these proteins into the bloodstream, allowing them to be measured to assess myocardial injury. The process of necrosis is not immediate in response to ischemia, and once necrosis occurs, it usually takes 1–4 hours for cTn to increase in the circulation before becoming detectable in the blood. This timing varies due to differences in analytical sensitivity of cTn assays, as well as differences in patients’ physiology.
Even with the widespread use of cTn assays worldwide, there remains some confusion among clinicians and laboratorians about the timing, frequency, and duration for measuring cTn after patients present with symptoms suggestive of ACS. Relying on the latest definition of MI, in this article we will discuss the way serial cTn ordering can be leveraged to support evidence-based clinical practices for efficient and cost-effective determinations of acute MI.
Serial cTn Testing Walkthrough
To illustrate the importance of serial cTn blood testing, we will use a case example. Consider the holiday season, when, due to family and work-related celebrations, individuals often delay seeing their doctors, even when they have acute ischemic symptoms such as chest, jaw, or arm pain. Once theses holiday procrastinators finally seek medical attention—often days after the initial event—their physicians may consider that they have had an acute MI, and order a work-up including an EKG and blood testing for cTn to assist in the differential diagnosis.
For our example, one holiday procrastinator, Ms. Minnesota, drives herself to the hospital approximately 3 days after her initial discomfort, because her chest pain still has not resolved. She arrives at the emergency department (ED) where her chief complaint is “shortness of breath and chest pain that worsens with exertion,” which all began 48 hours ago. Her clinician’s workup includes a cTn concentration along with other lab tests, an EKG, diagnostic imaging, and clinical history for the differential diagnosis of ACS.
Ms. Minnesota’s initial blood draw at presentation is considered time zero (0h). In this case, the clinical history of the exact time of the initial onset of chest pain is not clear in relation to the most recent acute event which brought the patient to the hospital. Since Ms. Minnesota presented with symptoms suggestive of ischemia, and had no previous medical history of stent placement, percutaneous coronary intervention (PCI), or coronary artery bypass graft (CABG), we’ll use the diagnostic criteria for MI following the Universal Definition guideline (See Box, right).
|
The Importance of Defining MI
|
Given the prominent role that cardiac troponin (cTn) plays in the definition of acute myocardial infarction (MI), any discussion of ordering—be it frequency, duration, or interpretation—should start with the current definition of acute MI.
The Third Universal Definition of Acute Myocardial Infarction, described by the Global Task Force (2012), states that in a clinical setting with evidence of myocardial necrosis consistent with acute ischemia, any one of the following criteria meets the diagnosis for an acute MI:
1) Detection of a rise and/or fall of cardiac biomarker values with at least one value above the 99th percentile upper reference limit (URL), with at least one additional finding of ischemic symptoms, such as (presumed) new significant ST-segment-T wave changes, new left bundle branch block (LBBB), pathological Q wave changes, imaging evidence of new loss of viable myocardium or new regional wall motion abnormality, or evidence of intracoronary thrombus by angiography or autopsy (type 1 MI).
2) Cardiac death with symptoms suggestive of myocardial ischemia and presumed new ischemic EKG changes, or new LBBB, but death occurred before cardiac biomarkers were obtained or would be increased (type 3 MI).
3) Related to percutaneous coronary intervention, where cTn values are elevated above the 99th percentile (normal baseline) or exceed their baseline values >20% (elevated stable/falling baseline) which is accompanied by: symptoms of ischemia or new ischemic EKG changes; angiographic findings consistent with complications; or imaging evidence of new loss of viable myocardium or new wall motion abnormality (type 4 MI).
4) Related to stents, where thrombosis findings during coronary angiography or autopsy is accompanied by rise and/or fall of cardiac biomarkers with one value above 99th percentile URL (type 4 MI).
5) Related to coronary artery bypass grafting, where cardiac biomarker values are elevated (with normal baseline) accompanied by: new pathological Q waves or LBBB or angiographic findings of new occlusion of graft or native coronary artery; or imaging evidence of new loss of viable myocardium or new wall motion abnormality (type 5 MI).
These criteria describe circumstances in which ischemic changes are seen in a clinical scenario consistent with acute MI, meaning a combination of imaging/angiographic evidence, symptoms/signs of ischemia, and specific changes in cTn levels coalesce to constitute an acute MI. The clinical classification of type 2 MI, defined as MI secondary to ischemia due to increased oxygen demand or decreased supply, is also predicated on the measurement of cTn and is an important area of ongoing study. Type 2 MI is not an acute coronary syndrome diagnosis, but may have an ST-segment elevation on EKG. Creatine kinase (CK)-MB should be briefly mentioned here for its historical importance. While it has previously contributed to assessments of acute MI, the 2012 Universal Definition clearly indicates that CK-MB is no longer appropriate for current clinical practice.
|
Without a diagnostic EKG finding, the role of cTn testing using contemporary assays becomes an important standard for ruling in or out an MI. In Ms. Minnesota’s case, the first cTn result was found to be below the 99th percentile upper reference limit (URL). Does that mean she has been ruled out for an MI? In short, no. According to the Universal Definition, we cannot decide based on this one value alone: she might yet be within that 1–4 hour window where cTn levels can still be rising to detectable levels after myocardial necrosis. Regardless of whether the initial cTn result is normal or increased above the 99th percentile value, the critical component for the diagnosis of an acute MI requires a directional trend in cTn values. This definition inherently requires at least two cTn results, which can display either a rising or falling pattern, over the initial 6–9 hours after a patient’s presentation (0h) with at least one value above the 99th percentile. This is why serial cTn orders, and not a single isolated cTn order, are essential to good clinical practice. Recent evidence has demonstrated that 6%–23% of adjudicated acute MI cases had normal concentrations of cTn, based on 4 different high sensitivity cTn assays, at presentation.
Using Timed Order Sets
At Hennepin County Medical Center, we recently instituted a single timed order set for cTnI, with pre-set collection times at 3-hour intervals following the initial cTnI blood draw in the ED. This translates into blood draws at 0, 3, 6, and 9 hours. We also follow this practice for all in-patient cTnI orders initiated within the hospital. If the patient originates in the ED as an outpatient, and is then admitted to a unit as an in-patient, the cTnI order set follows the patient regardless of his or her movement between departments within the hospital. This creates an environment in which we minimize missed cTnI collections, so that we treat patients appropriately, as early as possible, with a rule-in or rule-out MI determination.
The serial order set also comes into play if the clinician suspects a reinfarction during a patient’s hospitalization, initiating another timed order set of 0, 3, 6, and 9 hours. Evidence has proven that creatine kinase MB plays no role in detection of reinfarction. Time zero (0h) would coincide with recognition of a suspected reinfarction, or after an intervention, such as PCI, stent placement, or CABG procedure described by Universal Definition type 3, 4, and 5 MIs, respectively. The lower panel of Figure 1 illustrates how we triage patients with the cTn serial timed order set within different hospital locations, including a second order set cycle if a reinfarction is suspected. If a clinician orders a second timed order set, this triggers a quality assurance monitor for excessive cTn use. A pop-up box appears in the electronic health record and asks the following: Do you suspect a new MI? If not, why are you ordering a second order set? For a clinician to be able to obtain a new set of orders, both these questions need to be answered.
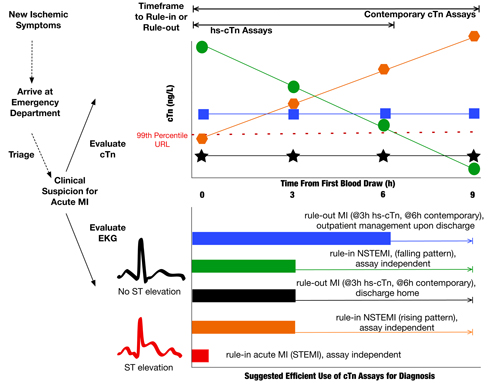
Looking forward to when we implement high-sensitivity cTn assays (hs-cTn), ruling out an MI then will tighten the timeframe. When presenting to the ED, patients, such as our Ms. Minnesota, will typically display one of the following cTn patterns shown in the upper panel of Figure 1, where cTn levels are rising, falling, or staying relatively flat—either below or above the 99th percentile. Based on our order set at Hennepin, if the patient’s initial two hs-cTn results at 0h and 3h are below the 99th percentile URL, and there is no diagnostic EKG or imaging finding, this would be sufficient to rule out MI in an otherwise low-risk patient; providing the ability to cancel subsequent outstanding 6- and 9-hour orders. In this way, an earlier rule-out can mean significant savings for the hospital through the possibility of an earlier discharge from the ED.
Similarly, contemporary cTn results can provide clear evidence for a directional trend after the second 3-hour value, with supporting EKG or imaging findings, to rule-in a patient, and additional cTn values would not be diagnostically additive or even necessary. However, given the imprecision at the 99th percentile for the assays currently used in laboratory practice in the United States, there may not be a clear rising or falling pattern by the second, 3h cTn test result, thus making the remaining 6h or 9h cTn results crucial for diagnosis. As a result, the outstanding orders generated with a serial, timed order set allow continuous sampling over the remaining 6 to 9 hour window, regardless of where the patient is receiving care within the hospital, be it the ED, coronary care unit, or elsewhere. It is important to note, however, that the coefficient of variation (%CV) is often >20% for contemporary assay performance in the real world, not quite as good as the values found in package inserts.
Unfortunately, many institutions often overlook the cost-ineffective clinical practice of “continuous cTn orders” after a patient has been ruled in for an MI. In such cases, clinicians typically order additional cTn testing either to look for a peak cTn concentration or to document that it has normalized below the 99th percentile value. Neither of these wasteful ordering habits is evidence-based, and they do not support or improve patient care. For example, based on Ms. Minnesota’s initial two cTnI results, without a clear cTn trend, our hypothetical patient is being admitted for further observation and continued diagnostic work up.
What to Look for After FDA Acts
If and when the Food and Drug Administration (FDA) clears hs-cTn assays, which could be as early as 2014, we expect to see a variety of changes. First will be distinct 99th percentile values for men (higher URL) and women (lower URL). Second, greater than 80% of normal subjects will have a measureable cTn above the limit of detection. Third will be a decreased clinical specificity compared with contemporary assays, with anticipated corresponding increases in cardiology consults. This is due to the fact that hs-cTn assays detect smaller amounts of myocardial injury in a larger number of pathologies associated with cardiovascular disease and injury.
These changes in analytical sensitivity—improved earlier clinical sensitivity (good for emergency medicine) and decreased clinical specificity (not so great for medicine/cardiology)—will potentially lead to new algorithms for ACS rule-in and rule-out, and changes in risk stratification assessment models in ACS and for general population screening and disease prevention. We also expect to see more new patients being evaluated for cardiac care follow-up in non-urgent care clinics.
Recent work from Europe, where hs-cTnI and T assays are already available, suggests that a single measurement of cTn at presentation for ACS rule-outs is not clinically appropriate: at least two values measured 2–3 hours apart after presentation, are still required. When hs-cTn assays gain FDA clearance for use in the U.S., further studies will be needed to assess the possible adjustments to serial cTn testing to effectively, efficiently, and accurately rule patients in or out for ACS and acute MI. How cTn orders are initiated in the ED and whether cTn testing becomes utilized more judiciously in the ED will require education by a team approach, including the laboratory, cardiology, and emergency medicine.
As the story of Ms. Minnesota illustrates, serial cTn ordering is a critical component of acute MI diagnosis readily understood in terms of the timing, frequency, and duration of cTn measurements. Overall, our suggested standard ordering practice, at 2–3 hour intervals over the 6–9 hours following presentation, can be readily implemented throughout a medical center, allowing for efficient patient diagnosis and care, and the potential for substantial cost savings.
SUGGESTED READING
Sandoval Y, Apple FS. The global need to define normality: The 99th percentile value of cardiac troponin. Clin Chem 2014;60:455–62.
Thygesen K, Alpert JS, Jaffe AS, et al.: the Writing Group on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. J Am Coll Cardiol 2012;60:1581–98.
Apple FS, Ler R, Murakami MM. Determination of 19 cardiac troponin I and T assay 99th percentile values from a common presumably healthy population. Clin Chem 2012;58:1574–81.
Apple FS, Collinson PO, IFCC Task Force on Clinical Applications of Cardiac Biomarkers. Analytical characteristics of high-sensitivity cardiac troponin assays. Clin Chem 2012;58:54–61.
Kavsak PA, Allen LC, Apple FS, et al. Cardiac troponin testing in the acute care setting: Ordering, reporting, and high sensitivity assays—an update from the Canadian Society of Clinical Chemists (CSCC). Clin Biochem 2011;44:1273–7.
Mills NL, Lee KK, McAllister DA, et al. Implications of lowering threshold of plasma troponin concentration in diagnosis of myocardial infarction: Cohort study. BMJ 2012;344:e1533.
Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med 2009;361:858–67.
de Lemos JA, Drazner MH, Omland T, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA 2010;304:2503–12.
 Sara Love, PhD, is a Clinical Chemistry Fellow at Hennepin County Medical Center in Minneapolis, Minn.
Sara Love, PhD, is a Clinical Chemistry Fellow at Hennepin County Medical Center in Minneapolis, Minn.
 Fred Apple, PhD, DABCC, is medical director of Clinical Laboratories at Hennepin County Medical Center and professor of laboratory medicine and pathology at the University of Minnesota School of Medicine in Minneapolis, Minn.Disclosures: Dr. Apple receives grant support, non-salary, from a majority of manufacturers of cTn assay, has served as a paid consultant for T2 Biosystems and Instrumentation Laboratory, and sits on the scientific/medical advisory board of the following companies: Phillips, Abbott Diagnsotics, and Instrumentation Laboratory.
Fred Apple, PhD, DABCC, is medical director of Clinical Laboratories at Hennepin County Medical Center and professor of laboratory medicine and pathology at the University of Minnesota School of Medicine in Minneapolis, Minn.Disclosures: Dr. Apple receives grant support, non-salary, from a majority of manufacturers of cTn assay, has served as a paid consultant for T2 Biosystems and Instrumentation Laboratory, and sits on the scientific/medical advisory board of the following companies: Phillips, Abbott Diagnsotics, and Instrumentation Laboratory.