Immune response directed against "self" is referred to as autoimmunity. The 1908 Nobel Laureate, Paul Ehrlich, first envisioned the notion of autoimmunity, which he termed "horror autotoxicus" (1). Ehrlich's pioneering theory considered that the immune system normally targets foreign substances and has an inbuilt tendency to avoid attacking self-tissues. However, when this process goes wrong, the immune system attacks self-tissues, resulting in autoimmune diseases (AID).
AID are a spectrum of diseases ranging from organ-specific, in which the immune system reacts against self-antigens in a particular tissue, to systemic, in which the immune response takes place against a specific antigen or antigens of multiple tissues. AID are a significant health concern throughout the world. Many of these diseases tend to be difficult or impossible to cure, for the obvious reason that the target of the immune response, which is self-antigens, cannot be eliminated. The chronic nature of AID has a significant impact on medical care utilization, direct and indirect economic costs, and quality of life. AID are among the leading causes of death among those younger than age 65 in the United States (2).
Laboratory Investigation of Autoimmune Diseases
AID are very difficult to diagnose, and the right treatment must be carefully chosen for the right disease at the right time. Each diagnosis requires a detailed history, physical exam, and often multiple laboratory tests. Laboratory testing is of great value when evaluating a patient with a suspected AID. However, not a single laboratory test establishes such a diagnosis. Typically, the diagnostic work-up involves multiple tests, including complete blood count, inflammatory markers, flow cytometry, and autoantibodies. Circulating autoantibodies against a wide number of structural and functional molecules that present in ubiquitous or tissue-specific cells also are considered valuable markers of AID. Detection of autoantibodies is an important component of the diagnostic criteria for AID, helps anticipate the clinical phenotype of individual patients, and contributes to assessment of overall disease activity.
Anti-Nuclear Antibody Testing
Anti-nuclear antibodies (ANA) are hallmarks of many autoimmune connective tissue diseases. The term ANA is now obsolete and even puzzling, as this historical label has come to include antibodies directed at various cellular compartments including nuclear constituents, components of the nuclear envelope, mitotic spindle apparatus, cytosol, cytoplasmic organelles, and cell membranes. ANA testing is used extensively for diagnosing and monitoring various AID such as systemic lupus erythematosus (SLE), Sjogren's syndrome, scleroderma, mixed connective tissue disease, polymyositis, and dermatomyositis. Development of the first method to identify ANA is considered one of the milestones in the history of clinical immunology over the last 60 years. However, the methodologies for measuring ANA have changed substantially over the years in recognition of a wider spectrum of potentially relevant autoantibodies and a sensible requirement to improve test performance and efficiency.
Both microscopy and immunoassay-based methods have significant importance in laboratory medicine. The traditional methods for detecting ANA are indirect immunofluorescence (IIF) and enzyme immunoassay (ELISA) (Figure 1).
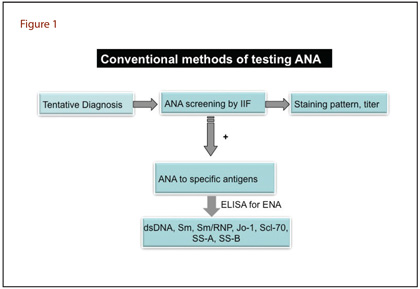
Indirect Immunofluorescence Assay
IIF assays to probe nuclear antigens were introduced more than 50 years ago, and this method still is the most widely used conventional technique for detecting ANA (3). The IIF method detects a large number of autoantibodies that bind to a variety of nuclear antigens mentioned earlier (Figure 2). Briefly, IIF involves placing patient serum on a slide containing tissue or HEp-2 cells (a human laryngeal epithelial cell line). ANAs bind to the specific antigen and after washing, the next step is to add fluorescein-labeled anti-human IgG. Laboratorians view the slide under a fluorescence microscope and report results with staining pattern and titer. Common patterns reflecting the types of antigens include homogenous, speckled, centromere, or nucleolar, all of which suggest associations with certain autoimmune diseases. IIF is highly sensitive and has broad screening potential but is not without limitations. Aside from a high false positive rate, IIF is time-consuming, laborious, and frequently yields discrepant inter-laboratory results. Moreover, IIF requires highly trained personnel and is hard to standardize because of the subjectivity of interpretation (4).
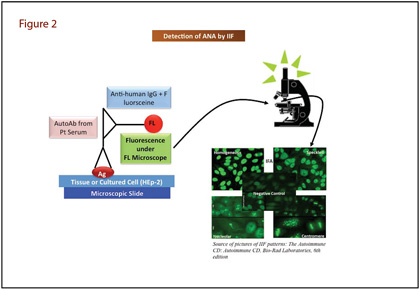
Since IIF still is recommended as the gold standard for ANA screening, in vitro diagnostics manufacturers have introduced automation to compensate for IIF's drawbacks. These automated systems offer significant improvements in slide preparation, and feature software to read, analyze, and interpret slides of digital images. Several companies offer these systems, including Nova View (Instrumentation Laboratory, Spain), Aklides (Medipan, Germany), Euroscope-Europattern (Euroimmun, Germany) and Zenit G Sight (A. Menarini Diagnostics, Italy). The automated systems demonstrate very high agreement with the manual method (5). Automated IIF systems might enhance ANA testing standardization and help reduce inter-laboratory variability. However, the laboratory community needs further evidence to determine whether this system efficiently identifies antigens of clinical significance and whether the different automated systems have an appropriate level of pattern recognition agreement.
ELISA
A positive IIF result leads to further investigations using ELISA to detect specific markers such as anti-dsDNA antibodies and/or anti-extractable nuclear antigens (anti-ENA) antibodies. ELISA uses specific nuclear antigens (SS-A, SS-B, Sm, Sm/RNP, Jo-1, and Scl-70) coated on a multi-well plate to detect antibodies in patient's serum. Sera samples are then added to the wells and ANA, if present, bind to the wells. Following washing, labeled ligand is added to the wells to detect bound antibody. The concentration of antibody is determined by comparison to a standard curve generated by known concentrations of ANA.
ELISA is automated, and in comparison to IIF, reduces the number of subjective measurements. However, it also has several disadvantages, including substrate differences (many sources of mammalian and non-mammalian DNA are used), differences in isotypes detected (IgG, IgA, IgM, or any combination), and antibody affinity. In the latter case, high affinity anti-dsDNA might be more relevant to SLE pathogenesis, but ELISA method detects both high and low affinity anti-dsDNA antibodies. ELISA also is subject to contamination with ssDNA, which causes overestimation of anti-dsDNA, but ssDNA antibodies are not specific for SLE. Finally, ELISA requires multiple assays for different antibodies.
Multiplex Immunoassay
Recent advances in protein identification methods have generated a reservoir of candidate biomolecules, thus creating an arcade for high-throughput multiplex immunoassays that allow simultaneous quantification of many analytes. Multiplex immunoassays yield abundant information on multiple proteins in diverse biological processes, thereby providing clinicians and scientists with insight into the identification and assessment of disease progression.
Two basic assay formats have been developed to facilitate simultaneous quantification of multiple antigens: planar array assays and microbead assays. Planar array assays spot different capture ligands at defined positions on a two-dimensional array. Microbead assays like those based on Luminex MAP technology involve multiple microbeads, each coated with different capture ligands. Flow cytometry then detects an assay-specific fluorescent signal, which allows simultaneous detection of multiple analytes in a single reaction. There is abundant evidence suggesting that automated Luminex-based systems can rapidly and efficiently determine a profile of multiple antibodies.
Recently, clinical diagnostic laboratories have introduced this technological advancement in multiplex immunoassay for simultaneous multiple analysis. Several studies suggest that this assay is a useful tool for detecting ANA in autoimmune diseases (5). Currently available commercial systems based on fluorescent microbeads technology include BioPlex 2200 (Bio-Rad Laboratories, Hercules, California), AtheNA Multi-Lite (Zeus Diagnostics, Raritan, New Jersey), QuantaPlex (Instrumentation Laboratory, Barcelona, Spain), and FIDIS (BioMedical Diagnostics, Marne la Vallee, France).
Table 1
| ANTIBODY |
TARGETED CONNECTIVE TISSUE AUTOIMMUNE DISEASES |
| dsDNA |
Systemic Lupus Erythematosus, Mixed Connective Tissue Disease, Scleroderma |
| SSA-60 kD |
Sjogren's syndrome, Systemic Lupus Erythematosus, Polymyositis, Scleroderma, Mixed Connective Tissue Disease |
| SSA-52kD |
Sjogren's syndrome, Systemic Lupus Erythematosus, Polymyositis, Scleroderma, Mixed Connective Tissue Disease |
| SSB |
Sjogren's syndrome, Systemic Lupus Erythematosus, Scleroderma |
| Sm |
Systemic Lupus Erythematosus, Mixed Connective Tissue Disease |
| Sm/RNP |
Mixed Connective Tissue Disease, Systemic Lupus Erythematosus |
| RNP-A |
Mixed Connective Tissue Disease, Systemic Lupus Erythematosus |
| RNP-60kD |
Mixed Connective Tissue Disease, Systemic Lupus Erythematosus |
| Chromatin |
Systemic Lupus Erythematosus, Mixed Connective Tissue Disease, Sjogren's syndrome, Polymyositis, Scleroderma |
| Scl-70 |
Scleroderma, Mixed Connective Tissue Disease |
| Centromere B |
Scleroderma, Systemic Lupus Erythematosus |
| Ribosomal P |
Scleroderma, Systemic Lupus Erythematosus |
| Jo-1 |
Polymyositis |
Experiences With a Multiplex Platform
Over the last 5 years our laboratory has been using one of these multiplex systems, BioPlex 2200, to test ANA. This is a fully automated Luminex-based system developed for high-throughput simultaneous analysis of 13 autoimmune analytes in a single tube, reacting with SSA (52 and 60 kDa), SSB, Sm, Sm/RNP, RNP-A, RNP-68 kDa, Scl70, centromere B, dsDNA, chromatin, Jo1, and ribosomal P proteins (Table 1). This system couples microspheres with different laser-reactive colors to antigens of interest and combines them in a single microtitre well. The specimen and a fluorochrome-coupled secondary antibody are then added. The analysis uses flow dual laser cytometry in which the first laser identifies the colour of the bead (addressing the identity of the coated antigen) and the second identifies the presence and quantity of autoantibody bound to the antigen (Figure 3). Among the 13 antibodies, anti-dsDNA antibody is calibrated against World Health Organization Wo/80 standard, and the results are quantitative and expressed in terms of IU/mL. Values ≥10 IU/mL are taken as positive. All other antibody results are semi-quantitative, expressed in terms of Antibody Index (AI), and values ≥1.0 AI are taken as positive. We report ANA screen as negative if the results for all 13 autoantibodies are negative. Conversely, if any of the 13 autoantibodies is positive, we report a positive ANA screen and show the AI of individual antibodies. The medical decision support software (MDSS) included in the system suggests a disease association based on how individuals with similar autoantibody values have been diagnosed. The MDSS uses a database of 1,200-plus real-world individuals and contains the specific ANA screen results (all 13 auto-antibodies) plus the autoimmune disease diagnosis as determined by a physician (if disease was present) for each individual included.
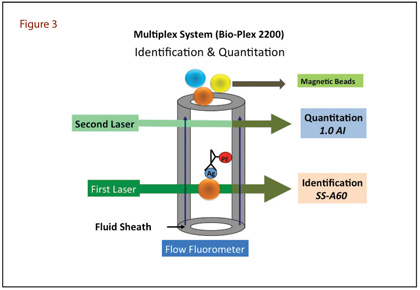
The BioPlex system has been successfully evaluated in several studies for detection of ANA. One study assessed the positivity rate of 510 samples collected from a cohort of apparently healthy blood bank donors and reported a positivity rate per individual analyte ranging from 0.0% to 1.8%, which translated into individual analyte specificity ranging from 98.2% to 100% (6). Another study of a retrospective hospital cohort of 1,104 patient samples demonstrated that this system was suitable as a sensitive screening test to confirm or to exclude the presence of a large number of autoantibodies simultaneously and rapidly (7).
A third, retrospective study analyzed 385 highly characterized antibody-positive sera and 196 negative sera (3). Overall sensitivity was 94.5% and specificity was 99.2%. A recent study using this multiplex system showed a higher sensitivity and a parallel specificity of the ANA-IIF (at 1:80 dilution) compared to the ANA screen (5). In addition, another study conducted for comparison of three anti-dsDNA testings demonstrated that BioPlex-multiplex and Farrzyme assays had similar overall agreement with the Farr assay, with BioPlex best reflecting disease activity in SLE patients (8).
A multicenter, prospective clinical study demonstrated that agreement between multiplex and EIA testing ranged from 99% (95% CI 98–100%) for Jo-1 to 70% (95% CI 76–82%) for ANA. The MDSS algorithm suggested an appropriate disease association in 75–100% of patients with SLE (9). These findings suggest that patterns of autoantibodies detected by this system, when analyzed by an interpretative algorithm, are useful in the evaluation of patients with autoimmune disorders.
This system offers the advantages of being automated, having a shorter turn-around time, detecting multiple antibodies simultaneously, and being less subjective. On the downside, by employing a limited number of antigens it can lead to false-negative results compared with IIF testing (13). This is associated particularly with incompetence in detecting antibodies related to some specific disease profiles such as autoimmune liver diseases. In addition, recombinant proteins used in the assay may be poorly recognized by human autoantibodies, leading to false-negative results.
When our laboratory introduced this multiplex system in assessing ANA, we faced challenges with both hospital physicians and external clients in accepting a new style of reporting as well as the analytical process. A particular concern was that the new testing system did not provide an IIF pattern and titer. That led us to compare the new method with IIF for detecting ANA in 239 consecutive serum samples from our immunology clinic and to investigate the clinical significance of discrepant results (10). The new method showed 82.2% positive agreement, 83.7% negative agreement, and 83.3% overall agreement. Among 13 new method negative/IIF positive patients, only three showed a significant titer (>1:80) in IIF. These three were clinically stable on treatment. Twenty-seven patients showed new method positive/IIF negative results, of which 22 had been diagnosed with various AID, two had not been diagnosed with an AID, and the medical records of the other three patients were not accessible. A number of patients showed positive anti-double stranded DNA (5/22), anti-SS-A (9/22), and anti-SS-B (6/22) results in the new method screen while these were missed by IFA.
Thus ANA testing with 13 selected antigens shows substantial agreement with IIF and appears to be a rapid, reliable tool for detecting ANA and displays overall comparability to IIF. The study's findings helped our laboratory and clinics to accept the results of the new method. In addition, with continued communication regarding the principle of the new method and interpretation of results, the physicians in our hospital and referral laboratories became familiar with this new system.
We average about 70–80 specimens a day, and all the procedures are completed and reported within 90 minutes compared to the 7–8 hours required for setting up the test manually, obtaining results, and reporting. In situations where there is a strong clinical suspicion of underlying systemic autoimmune disease, but where the ANA screen by multiplex method produces negative results, we arrange additional IIF tests.
Conclusions
Multiplex technology represents a real improvement of the diagnostic power of autoantibody testing. ANA testing by multiplexing has good concordance with the comparative methods. This multiplex system represents a rapid, sensitive, and specific method for diagnosing AID, particularly when the specificities of individual antibodies and the combinations of antibodies are taken into consideration. In laboratories with a large daily volume of samples, the labor savings and absence of subjective error in interpretation of results associated with this approach could result in significant savings and quality improvement. The physician receives results faster and gets the results of additional antibodies compared to the conventional ELISA method. In the future, inclusion of additional antigens associated with the nucleolar pattern and autoimmune liver disease would further enhance the capability of this system in detecting ANA.
Acknowledgements
Thanks to all the members of the Immunology Section of Clinical Chemistry and Immunology Division of Hamilton Regional Laboratory Medicine Program (HRLMP). Special thanks to Debra Collins, medical laboratory technologist, HRLMP; Ed Young, PhD, professor emeritus, McMaster University; and Paul Keith, MD, clinical immunologist, Hamilton Health Sciences and McMaster University.
REFERENCES
- Bell E, Bird E. Autoimmunity. Nature 2005;435:583.
- Walsh SR, Rau LM. Autoimmune diseases: A leading cause of death among young and middle-aged women in the United States. Am J Public Health 2000;90:1463–6.
- Binder SR. Autoantibody detection using multiplex technologies. Lupus 2006;15:412–21.
- Ulvestad E, Kanestrøm A, Madland TM, et al. Evaluation of diagnostic tests for antinuclear antibodies in rheumatological practice. Scand J Immunol 2000;52:309–15.
- Tozzoli R, Bonaguri C, Melegari A, et al. Current state of diagnostic technologies in the autoimmunology laboratory. Clin Chem Lab Med 2013;51:129–38.
- Shovman O, Gilburd B, Barzilai O, et al. Evaluation of the BioPlex 2200 ANA screen: Analysis of 510 healthy subjects: incidence of natural/predictive autoantibodies. Ann NY Acad Sci 2005;1050:380–8.
- Desplat-Jego S, Bardin N, Larida B, et al. Evaluation of the BioPlex 2200 ANA screen for the detection of antinuclear antibodies and comparison with conventional methods. Ann N Y Acad Sci 2007;1109:245–55.
- Venner AA, Ibañez D, Gladman DD, et al. Comparison of three anti-dsDNA assays: Performance and correlation with systemic lupus erythematosus disease activity. Clin Biochem 2013;46:317–20.
- Moder KG, Wener MH, Weisman MH, et al. Measurement of antinuclear antibodies by multiplex immunoassay: A prospective, multicenter clinical evaluation. J Rheumatol 2007;34:978–86.
- Sohn K-Y, Collin D, Keith PK, et al. Comparison between multiplex immunoassay and immunofluorescence methods in detection of anti-nuclear antibodies. Clin Biochem 2010;43:
783–4.
 Kun-Young Sohn, MD, PhD, is a clinical biochemist at the Trillium Health Center, Mississauga, Ontario and affiliated with the Department of Laboratory Medicine & Pathobiology, Faculty of Medicine, University of Toronto, Ontario, Canada.
Kun-Young Sohn, MD, PhD, is a clinical biochemist at the Trillium Health Center, Mississauga, Ontario and affiliated with the Department of Laboratory Medicine & Pathobiology, Faculty of Medicine, University of Toronto, Ontario, Canada.
 Waliul I. Khan, MBBS, PhD, FRCPath, is an immunologist at the Hamilton Regional Laboratory Medicine Program of Hamilton Health Sciences and St. Joseph Health Care, Hamilton, Ontario, Canada and an associate professor at the Department of Pathology & Molecular Medicine, Faculty of Health Science, McMaster University, Hamilton,
Waliul I. Khan, MBBS, PhD, FRCPath, is an immunologist at the Hamilton Regional Laboratory Medicine Program of Hamilton Health Sciences and St. Joseph Health Care, Hamilton, Ontario, Canada and an associate professor at the Department of Pathology & Molecular Medicine, Faculty of Health Science, McMaster University, Hamilton,
Ontario, Canada.