Until recently, gluten-related disease and celiac disease (CD) were considered to be virtually synonymous. The classical presentation of CD encompasses diarrhea, steatorrhea, growth failure, weight loss, malnutrition, abdominal bloating and pain, and edema due to hypoalbuminemia—all manifestations of malabsorption attributable to small bowel mucosal inflammation and villous atrophy triggered by dietary gluten.
However, it is increasingly evident that many, if not most individuals have 'non-classical' CD and, furthermore, that many patients have wheat- or gluten-related conditions that do not even fall under the diagnosis of CD. The resulting confusion in the scientific literature regarding the definition, diagnosis, and treatment of gluten-related conditions has hampered the clinical management of these conditions.
In addition, failure to recognize atypical, non-gastrointestinal symptoms has led to delayed or missed diagnoses of gluten-related disorders with the potential for increased morbidity and healthcare costs. Widespread media reports that wheat is harmful have compounded these challenges, leading to an almost indiscriminate adoption of gluten-free diets for a variety of non-specific gastrointestinal and systemic symptoms.
In an attempt to rationalize and standardize the definitions for gluten-related conditions, an international, multidisciplinary task force published the Oslo definitions for celiac disease and other gluten-related disorders (2). Using these definitions, this article will explain the differences among the various disorders and allergies associated with wheat and how laboratory tests should be utilized.
Gluten-Related Disorders
Gluten-related disorders (GRD) is the overarching term the Oslo group proposed to describe all gluten-triggered diseases (Figure 1). The Oslo group recommends that GRD replace "gluten intolerance," an imprecise term that potentially includes individuals with symptoms related to other grain constituents such as lectins or carbohydrates. GRD includes CD, non-celiac gluten sensitivity (NCGS), dermatitis herpetiformis (DH), and gluten ataxia.
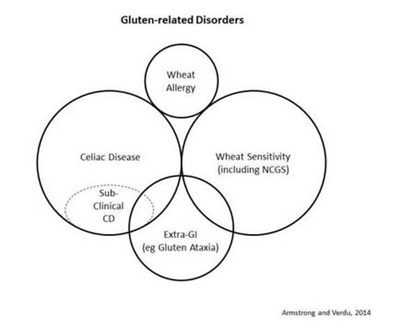
Figure 1
Celiac Disease
The Oslo group defined CD as "a chronic small intestinal immune-mediated enteropathy precipitated by exposure to dietary gluten in genetically predisposed individuals" (2). Classical CD is the term used to describe individuals with signs and symptoms of malabsorption, including diarrhea, steatorrhea, weight loss, or growth failure (2). Patients diagnosed with non-classical CD have no signs or symptoms of malabsorption, but they may present a variety of other gastrointestinal or systemic symptoms (Table 1).
Table 1 – Symptoms of 'Classical' and 'Non-Classical' Celiac Disease
The reported worldwide sero-prevalence of CD ranges from 0.3% to 1.2%, with a prevalence of about 1% in North America; however, most individuals with CD remain undiagnosed because of a low index of clinical suspicion and because many affected individuals do not have classical CD symptoms.
The sine qua non of classical CD is duodenal mucosal injury, progressing from increased intra-epithelial lymphocytes (IEL > 25 per 100 enterocytes) in an otherwise normal mucosa, through further increases in IEL density (> 40 per 100 enterocytes) with crypt hyperplasia, to total villous atrophy (3). Endoscopic biopsy remains the gold standard for CD diagnosis, but serological testing offers a quicker, more acceptable, and less expensive alternative.
Antibodies to gliadin were used first for the diagnosis of CD, but both immunoglobulin A (IgA) and immunoglobulin G (IgG) subclasses of anti-gliadin antibodies (AGA) have a low positive-predictive value for diagnosing CD and have been superseded by anti-endomysial antibodies (EMA) or anti-tissue transglutaminase (tTG) antibodies. Recent studies suggest that tests using IgA and IgG antibodies to deamidated gliadin peptide (DGP) perform comparably to EMA and tTG antibody tests for the diagnosis of CD (4, Figure 2).
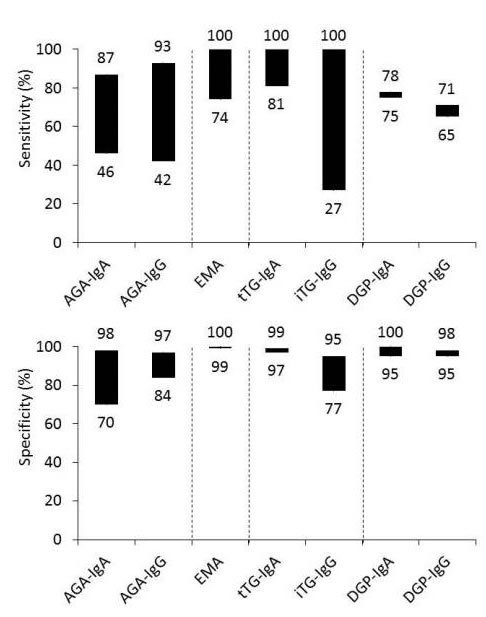
Figure 2
Both histological and serological testing have been validated for the diagnosis of classical CD in individuals who have histological features of mucosal injury: crypt hyperplasia (Marsh Type 2) and villous atrophy (Marsh Type 3). Some individuals with CD report no symptoms either because they truly have no symptoms or because they have become accustomed to chronic, low-grade symptoms. The preferred term is subclinical celiac disease (SCCD), rather than asymptomatic, silent, latent, or potential CD.
Patients with refractory CD have persistent signs or symptoms of malabsorption and villous atrophy despite strictly adhering to a GFD for more than 12 months after being definitively diagnosed with CD and cleared of malignancy and other causes of villous atrophy (2).
Wheat Sensitivity or Non-Celiac Gluten Sensitivity
Some individuals presenting clinically with functional bowel symptoms and no organic disease self-report wheat sensitivity. The wheat components able to cause symptoms include gluten and non-gluten proteins (5,6,7) as well as poorly digestible carbohydrates (8). Non-celiac gluten sensitivity (NCGS) implies a specific reactivity to gluten in those who do not have CD but who develop gastrointestinal or extra-gastrointestinal symptoms, immunological, or morphological changes after exposure to dietary gluten. NCGS is preferred to "gluten sensitivity."
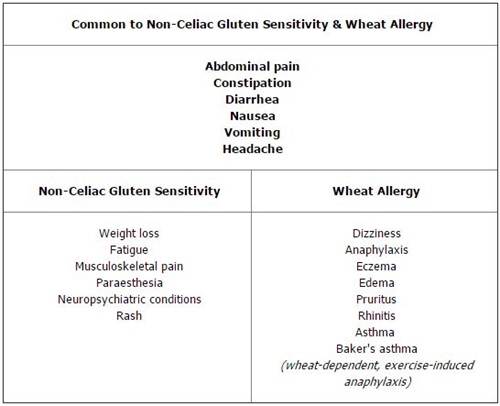
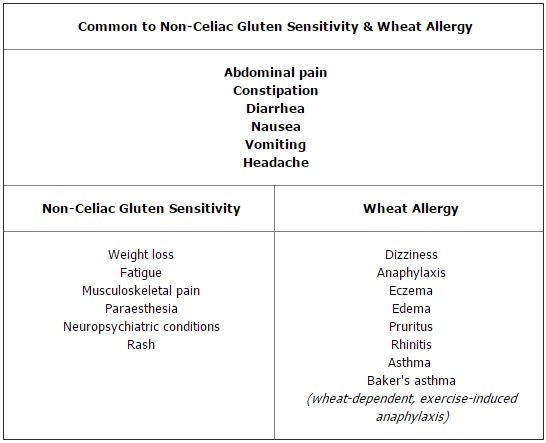
The prevalence of NCGS is unknown, although there are unsubstantiated suggestions that it may affect more than 5% of the population (9,10). NCGS may present with symptoms similar to those associated with CD (Table 2, online) and it may be associated with severe neuropsychiatric conditions such as cerebellar ataxia and schizophrenia (9). Although villous atrophy or a serological response to tTG, EMA, or DGP are not hallmarks of NCGS, gluten exposure may induce an innate immune response with increased IELs even in the absence of HLA-DQ2. A randomized, double-blind study of patients with NCGS reported that gluten exposure did, indeed, induce symptoms in those who had no histological or serological evidence of CD (11).
Impaired symptomatic tolerance of dietary gluten is not specific for CD and it may occur in individuals who are HLA-DQ2 negative, suggesting that the pathogenetic mechanisms underlying NCGS are different from those causing CD. Furthermore, a high proportion of HLA-DQ2-positive patients with self-identified gluten-related symptoms do not have CD although they have a greater symptom response to gluten challenge than patients who have CD (12).
NCGS is now widely accepted as being distinct from CD, but it remains a diagnosis of exclusion; there is no diagnostic test and no standard approach to making a diagnosis. Many individuals with a potential GRD will self-identify; they may have decided, independently or in consultation with an alternative healthcare practitioner, that their symptoms are caused by gluten, they may have a relative with CD or, increasingly, they may have used an over-the-counter, multiple food allergy serology kit. Other individuals with debilitating, non-specific gastrointestinal or systemic symptoms may undergo extensive investigations before being diagnosed with NCGS (10).
A clinical diagnosis of NCGS requires the exclusion of CD and wheat allergy. However, demonstrating a symptomatic response to a gluten challenge may be difficult in practice, as gluten would need to be purified to remove contaminants such as amylase trypsin inhibitors (ATI) which may cause symptoms independently of gluten (7). Although there are no specific serologic markers for NCGS, about 50% to 60% of patients are positive for IgA or IgG AGA (13,14). The finding of AGA in symptomatic individuals who do not have CD is suggestive, but not diagnostic of NCGS; conversely, the absence of AGA does not rule out NCGS. In theory, a double-blinded, placebo-controlled food challenge using purified gluten-containing capsules should identify individuals with gluten-induced symptoms but, in practice, this approach is impractical and unrewarding. Conversely, an open challenge with bread slices is subject to a substantial placebo effect. In many cases, patients themselves will decide whether or not to continue a GFD based on its effect on their symptoms and general well-being.
It is critical to recognize that the goals of diagnosis are not, necessarily, identical for research and clinical practice. For research, a precise definition of NCGS is important for studying the underlying mechanisms, whereas in clinical practice, accurate identification of the symptom triggers is important to minimize the patient's symptoms and minimize any harm arising from inappropriate or inadequate therapy. It is inappropriate to advise against a healthy, well-balanced GFD if this improves the individual's well-being, even if there is no objective evidence of CD or abnormal immunological response to gluten. Conversely, it is important to identify other remediable causes for the patient's symptoms such as dietary carbohydrate intolerance, lactose or fructose intolerance, inflammatory bowel disease, gastrointestinal dysmotility, pancreatic insufficiency, or irritable bowel syndrome (IBS).
Table 2 – Symptoms of Non-Celiac Gluten Sensitivity and Wheat Allergy
Non-Gluten-Related Disorders
CD and NCGS (Tables 1 and 2,) share non-specific symptoms with many other gastrointestinal tract disorders. Because non-specific gastrointestinal symptoms are common and because grains are ubiquitous in most diets, it is inevitable that many people will attribute their symptoms to dietary gluten even though gluten is not the only constituent of grain products that can cause gastrointestinal and systemic symptoms.
Wheat Allergy
Wheat allergy (WA) is an immune IgE-mediated reaction to gliadins found in wheat but not rye, barley, or oats. It is less common than CD, affecting about 0.1% of the general population and slightly more children, 0.4–1.0%. WA can cause symptoms not only similar to those of CD, but also affecting the upper aerodigestive tract (irritation, itching, and swelling), respiratory tract (anaphylaxis, shortness of breath, wheezing), and skin (hives, rash, swelling) (Table 2, online). WA usually develops in the pre-school years and, unlike CD, often resolves by time children reach age 7 (16). Strict avoidance of wheat products is key, but most patients can tolerate rye, barley, oats, and other grains, so they do not require a strict GFD.
Irritable Bowel Syndrome
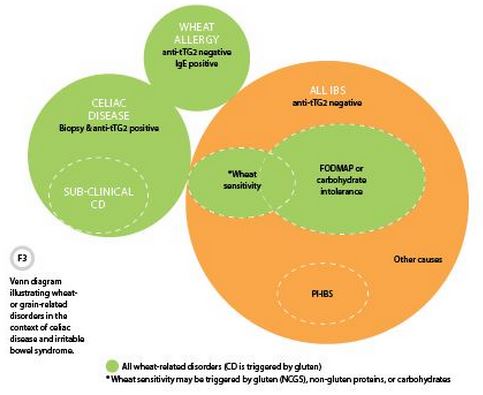
IBS is defined by chronic abdominal pain, variable bowel habit, and other gastrointestinal symptoms for which no organic or structural cause has been identified; thus, someone with a confirmed GRD does not have IBS (Figure 3). Reports that the association between CD and IBS is 4 to 5 times greater than would be expected by chance and that a GFD normalizes bowel habit and gastrointestinal symptoms in some patients with diarrhea-predominant IBS (13) indicate that gluten can cause IBS-like symptoms, but not that it causes IBS. IBS is a heterogeneous disorder with multiple etiologies, including many other dietary triggers. In patients with IBS symptoms, both CD and wheat sensitivity should be considered in the differential diagnosis.
Carbohydrate Intolerance
Grains and cereals are major sources of poorly-absorbed dietary carbohydrates that can cause gastrointestinal symptoms in patients with IBS and other functional gastrointestinal disorders. Gastrointestinal symptoms attributed to gluten may, therefore, be due to poorly-absorbed carbohydrates, including fermentable oligo-, di-, and mono-saccharides and polyols (FODMAPs) (8,17). Carefully designed clinical management protocols and research studies are needed to differentiate clearly between the effects of gluten and poorly-absorbed carbohydrates in the pathogenesis of gluten-related disorders and functional gastrointestinal disorders.
Diagnosis of Gluten-Related Disorders
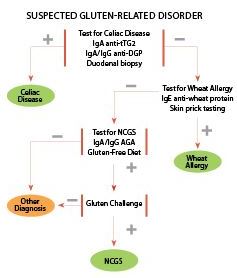
No consensus exists on how best to investigate suspected GRD. However, the diagnostic community generally agrees on the first step: excluding CD based on negative serology for IgA anti-tTG, IgA or IgG anti-DGP, and negative histology in someone taking a normal, gluten-containing diet. Serology may be negative in up to 10% of CD patients, and quantitative measurement of serum immunoglobulins should identify GRD patients with IgA deficiency in whom IgG anti-DGP antibodies or AGA may be detectable. Confirmation of CD or villous atrophy comes through endoscopy, with six duodenal biopsies (four from second part of duodenum; two from duodenal bulb). HLA typing may be helpful in equivocal cases, as the absence of HLA-DQ2 and HLA-DQ8 has a very high negative-predictive value for CD.
WA testing using IgE wheat protein antibodies and specific skin prick testing should be considered if tests for CD are negative, especially if there are other allergic symptoms (Table 2, online).
If CD and WA have been excluded, testing for IgA or IgG AGA may indicate the presence of NCGS but these tests may be negative in more than 50% of patients with typical symptoms (14). A diagnosis of wheat sensitivity or NCGS if AGA are positive requires a strict GFD for 6 to 12 months and documented clinically improved symptoms. In patients with positive serology, AGA titers may normalize. Relapse of symptoms following a purified gluten challenge—particularly if combined with a low FODMAPs diet—or resumption of a normal gluten-containing diet provides further support for a diagnosis of NCGS (11). HLA typing is not helpful to exclude NCGS or extra-gastrointestinal GRD, as many patients with DH or gluten ataxia will be negative for HLA-DQ2 or HLA-DQ8.
See Figure 4 for our summarized approach to the clinical investigation and diagnosis of gluten-related disorders, based on widely available tests.
References
- van Berge-Henegouwen GP, Mulder CJ. Pioneer in the gluten free diet: Willem-Karel Dicke (1905–1962), over 50 years of gluten free diet. Gut 1993;34:1473–5.
- Ludvigsson JF, Leffler DA, Bai JC, et al. The Oslo definitions for coeliac disease and related terms. Gut 2013;62:43–52.
- Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity ('celiac sprue'). Gastroenterology 1992;102:330–54.
- Armstrong D, Don-Wauchope AC, Verdu EF. Testing for gluten-related disorders in clinical practice: The role of serology in managing the spectrum of gluten sensitivity. Can J Gastroenterol 2011;25:193–7.
- Verdu EF, Armstrong D, Murray JA. Between celiac disease and irritable bowel syndrome: The "No Man's Land" of gluten sensitivity. Am J Gastroenterol 2009;104:1587–94.
- Vazquez-Roque MI, Camilleri M, Smyrk T, et al. A controlled trial of gluten-free diet in patient with irritable bowel syndrome-diarrhea: Effects on bowel frequency and intestinal function. Gastroenterology 2013;144:903–11.
- Junker Y, Zeissig S, Kim S-J, et al. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. J Exp Med 2012;209:2395–408.
- Biesiekierski JR, Peters SL, Newnham ED, et al. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology 2013;145:320–28.
- Lundin KEA, Alaedini A. Non-celiac gluten sensitivity. Gastrointest Endosc Clin N Am 2012;22:723–34.
- Anonymous, Rostami K, Hogg-Kollars S. A patient's journey. Non-coeliac gluten sensitivity. Br Med J 2013; 345:e7982.
- Biesiekierski JR, Newnham ED, Irving PM, et al. Gluten causes gastrointestinal symptoms in subjects without celiac disease: A double-blind randomized placebo-controlled trial. Am J Gastroenterol 2011;106: 508–14.
- Brottveit M, Vandvik PO, Wojniusz S, et al. Absence of somatization in non-coeliac gluten sensitivity. Scand J Gastroenterol 2012;47:770–77.
- Wahnschaffe U, Schulzke JD, Zeitz M, et al. Predictors of clinical response to gluten-free diet in patients diagnosed with diarrhea-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol 2007;5: 844–50.
- Volta U, Tovoli F, Cicola R, et al. J Clin Gastroenterol 2012;46:680–5.
- Hadjivassiliou M, Sanders DS, Grünewald RA, et al. Gluten sensitivity: From gut to brain. Lancet Neurol 2010;9:318–90.
- Keet CA, Matsui EC, Dhillon G, et al. The natural history of wheat allergy. Ann Allergy Asthma Immunol 2009; 102:410–5.
- Gibson PR, Shepherd SJ. Food choice as a key management strategy for functional gastrointestinal symptoms. Am J Gastroenterol 2012;107: 657–66.
David Armstrong, MA, MB BChir, FRCPC, FRCP(UK), is an associate professor of medicine at McMaster University and a consultant gastroenterologist at Hamilton Health Sciences in Hamilton, Ontario. +Email: [email protected]
Elena F. Verdú, MD, PhD, is the Canada Research Chair in Inflammation, Microbiota, and Nutrition and an associate professor in the department of medicine at McMaster University.