The American Cancer Society estimates that 22,280 new cases and 15,500 deaths from ovarian cancer occurred in the U.S. in 2012. The disease is the leading cause of death from gynecological cancers, as well as the fifth-leading cause of cancer deaths in women. Unlike breast cancer in which great strides have been made in detecting early-stage disease and saving lives, many women die of ovarian cancer because the early stages have no obvious symptoms and no screening tests have proven to be effective.
Today, only a limited number of tumor markers for ovarian cancer have been cleared by the U.S. Food and Drug Administration (FDA). In general, tumor marker applications range from screening and monitoring therapy response or recurrence to selecting treatment and predicting prognosis. This article will describe the currently available ovarian cancer serum markers and the clinical evidence supporting their use.
Origin and Classification
Approximately 88% of ovarian cancer cases present in women 45 years of age and older, with a median age of diagnosis of 63 years. Unfortunately, most cases are diagnosed in the late stages of the disease, which explains the poor survival rate. If diagnosed at stage 1, the 5-year survival rate is about 93%; however, only 15% of all cases are detected at this stage, and usually only incidentally during another medical procedure. Most women, approximately 63%, are diagnosed at stage 3 or higher. In these cases, the 5-year survival rate is only 27%.
Ovarian cancer is often thought of as a single disease; however, it is composed of several related, but distinct, tumor categories. Oncologists define three main types of tumors based on the type of cells that the tumor originates from: epithelium, germ cell, or stroma (Figure 1). The vast majority, 90%, of ovarian malignancies originate from epithelial cells.
Figure 1
Ovarian Cancer Types
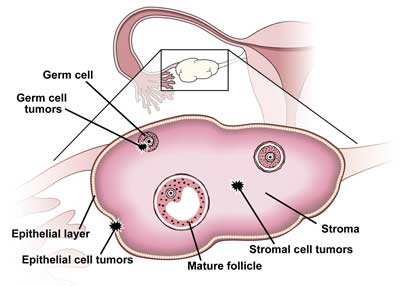
Epithelial ovarian tumors are further subdivided into five histological subtypes: serous, mucinous, endometrioid, clear cell, and transitional, and of these, epithelial serous carcinomas represent the majority of all primary ovarian carcinomas. Ovarian germ cell tumors develop from the cells that produce the ova or eggs, whereas ovarian stromal tumors are a rare class of malignancies that develop from connective tissue cells which hold the ovary together and produce the female hormones estrogen and progesterone. Germ cell and stromal tumors combined account for as few as 5–10% of all ovarian cancers.
The complex classification of ovarian cancers arises from their different clinical presentation, tumorgenesis, and gene expression profiles. Unfortunately, this complexity makes it challenging to identify and characterize effective biomarkers for ovarian cancer. Currently, the tumor markers described below are most useful for epithelial ovarian cancer.
Table 1
Clinical Applications of Serum Biomarkers for Ovarian Cancer |
| Uses |
CA125 |
HE4 |
OVA1 |
| Screening |
No |
No |
No |
| Discrimination of pelvic masses |
Yes* |
Yes+ |
Yes |
| Monitoring treatment |
Yes |
Yes |
No |
| Detection of recurrence |
Yes |
Yes |
No |
* As a component of the RMI or ROMA score
+ As a component of the ROMA score |
CA125: A Widely Used Marker
CA125, also known as mucin 16 (MUC16), is a cell-surface, glycoprotein antigen normally expressed in tissues derived from coelomic epithelia, such as ovary, fallopian tube, peritoneum, pleura, pericardium, colon, kidney, and stomach. The CA125 biomarker is FDA cleared as an aid in detecting residual or recurrent epithelial ovarian carcinoma in patients who have undergone first-line therapy. It is also indicated as an aid in monitoring response to therapy for patients with epithelial ovarian cancer.
About 80% of women with advanced ovarian cancer have elevated levels of CA125. However, the biomarker's sensitivity is poor in early stages of the disease, with an average of just 50% for stage I. The sensitivity increases to about 90% for stage II or greater. CA125 also lacks specificity, as patients with nongynecological cancers, including breast, colon, endometrial, and pancreatic, also have elevated levels of this analyte. Furthermore, levels are higher in a variety of benign conditions, such as endometriosis, uterine fibroids, pelvic inflammatory disease, heart failure, liver and renal disease, and can be elevated in as many as 1% of healthy women.
Due to its lack of sensitivity and specificity in a single determination, CA125 is not recommended for screening asymptomatic women. The Prostate, Lung, Colorectal, and Ovarian Cancer Screening (PLCO) trial conducted the most recent randomized, controlled trial on the utility of annual screening with CA125 and its impact on ovarian cancer mortality. In this trial, researchers randomly assigned 78,237 healthy women age 55–74 years to a group that had annual screening tests with CA125 and transvaginal ultrasound or to the usual care control group. Screening identified more ovarian cancers—212 compared with 176 found in women who received usual care—but the researchers did not observe any difference in cancer staging between the groups. Screening also did not reduce ovarian cancer mortality: 118 women died of ovarian cancer in the screened group compared with 100 in the usual care group.
Furthermore, screening resulted in unnecessary surgeries due to false-positive test results. Of the 10% of women in the screening group who had a false-positive result, 33% had an oophorectomy. Of that group, 15% also had a serious complication, such as an infection, surgical complication, or a cardiovascular or pulmonary event.
These findings from the PLCO trial further support the longstanding position of various professional entities that ovarian cancer screening has little effect on reducing mortality rates while at the same time increasing the risk of harm in patients with low risk of ovarian cancer. However, professional organizations do recommend that women with a history of hereditary ovarian cancer should be screened every 6 months with CA125 and transvaginal ultrasonography. These women have an estimated 40% lifetime risk of developing the disease, so early intervention may benefit them.
Unfortunately, clinicians' understanding of the clinical utility of CA125 as a screening test is somewhat alarming, as demonstrated by two recent studies that surveyed 2,338 physicians, including family practitioners, general internists, and obstetrician-gynecologists, about the effectiveness of the biomarker and transvaginal ultrasound in asymptomatic women in the U.S. with an average risk of developing ovarian cancer. In one study, 40% of responders believed that CA125 and annual transvaginal ultrasound were effective ovarian cancer screens, whereas only 28% responded that neither approach was an effective ovarian cancer screen. In addition, 28% of physicians said they would offer screening tests to low-risk women.
In contrast to its poor utility as a screening biomarker, studies show that CA125 is useful as an aid in assessing the risk of a malignant versus benign tumor in women who present with an adnexal mass. Identifying women at higher risk of malignancy not only enables clinicians to triage patients who will benefit from more specialized care, but it also decreases morbidity and mortality and increases overall survival in the patients referred for surgical intervention. In particular, CA125 concentrations >95 U/mL in postmenopausal women may help distinguish benign from malignant masses.
CA125 combined with sonographic features of the adnexal mass is another tool for risk stratification. The risk of malignancy index (RMI) uses the CA125 concentration multiplied by menopausal status and ultrasound score for risk calculation. Clinicians frequently use cutoffs of 200 or 250 and refer patients with higher scores to specialized gynecology/oncology teams. Researchers have reported the sensitivity and specificity of this approach as 85% and 92%, respectively, but clearly the scoring system relies heavily on the expertise of the sonographer.
FDA initially cleared CA125 assays for predicting the likelihood that women previously diagnosed with ovarian cancer would have evidence of residual tumor after surgical intervention. In fact, studies show residual disease is highly likely (>95% accuracy) in women with CA125 levels >35 U/mL who have had surgery to remove a tumor. Generally speaking, these women are referred to chemotherapy.
Oncologists also commonly use CA125 to monitor patients' response to chemotherapy. Although there are no specific guidelines, they typically test patients about 2 weeks before initiating treatment, 2–4 weeks afterwards, and every 2–3 weeks during follow-up. For long-term follow-up, current guidelines suggest monitoring every 2–4 months for 2 years and then less frequently if there is no evidence of disease.
CA125 is available on many automated immunoassay platforms, and manufacturers use similar reference values (<35 U/mL). However, results of CA125 measurements in both patients and proficiency testing materials often vary significantly depending on the assay used. This is likely due to differences in manufacturers' calibration, assay design, and antibodies' specificities. Due to these differences, it is important to reestablish baseline levels in patients who are serially monitored if the lab switches from one manufacturer to another.
HE4: The Newest Serum Marker
Human epididymis protein 4 (HE4) belongs to the family of whey acidic four-disulfide core (WFDC) proteins. Researchers first identified HE4 in the epithelium of the distal epididymis and originally thought it was a protease inhibitor involved in sperm maturation. To date, however, researchers have not identified HE4's biological function.
Studies show that HE4 is overexpressed in 93% of serous, 100% of endometrioid, and 50% of clear cell tumors, but not in mucinous ovarian carcinomas. In one study of 233 patients with a pelvic mass, including 67 with epithelial ovarian cancer, HE4 had a higher sensitivity than CA125, 72.9% vs. 43.3%, respectively, at a specificity of 95%. Researchers also found HE4 to be elevated in more than half of the ovarian cancer patients who did not have elevated CA125 levels; therefore, the combination of markers provided slightly improved sensitivity.
In June 2008, FDA cleared Fujirebio Diagnostics' HE4 immunoassay as an aid in monitoring recurrence or disease progression in patients with epithelial ovarian cancer. This assay has not been approved as a screening test for ovarian cancer in asymptomatic women. Unlike CA125, HE4 is not yet widely available on automated immunoassay platforms. As assay availability increases, however, using the same antibodies as those in the original FDA-cleared assay should help intra-assay comparability.
Today, clinicians use CA125 and HE4 in combination as an aid in assessing the likelihood of finding a malignancy during surgery in women presenting with a pelvic mass. The Risk of Ovarian Malignancy Algorithm (ROMA) takes into consideration the concentration of the two analytes and the patient's menopausal status to generate a score on a scale of 0–10, which translates to a high or low likelihood of finding a malignancy based on established cutoffs. Women with ROMA scores above the cutoff have an increased risk of ovarian cancer, and should be referred to a gynecological oncologist prior to surgery.
ROMA scores alone are not sufficient for triaging patients; other clinical information should also be incorporated into the assessment. For example, a woman's menstrual status is an important consideration. At a specificity of 75%, the cutoff values have reported sensitivities of 77–81% for premenopausal women and 90–92% for postmenopausal women.
It is important to note that so far FDA has granted clearance only to the combination of Fujirebio's HE4 assay and Abbott's ARCHITECT CA125 II assay for calculating ROMA scores. Laboratories that want to use a combination of assays from other manufacturers need to establish appropriate cutoff values after conducting their own validations. It is best to include ROMA scores with the laboratory findings because ROMA score calculators are available on the Internet and are often marketed directly to clinicians. Laboratorians should discourage clinicians from using such calculators. Not only are clinicians usually unaware of the specific assays used by the laboratory, but they also risk making an inaccurate diagnosis if the wrong cutoff value is used.
OVA1: A Multi-Analyte Assay for Risk Stratification
The OVA1 test from Vermillion, Inc. measures the serum levels of five analytes—CA125, transthyretin (prealbumin), apolipoprotein A1, beta2 microglobulin, and transferrin—using two different immunoassay platforms, the Roche Elecsys for CA125 and the Siemens BNII for the four other analytes. The company's proprietary software, OvaCalc, uses the values of these analytes along with the woman's menopausal status to calculate a single score. A high probability of malignancy is defined as a score of ≥5.0 in premenopausal women and ≥4.4 in postmenopausal women. Although an OVA1 score above the cutoff is not a diagnosis of cancer, it is indicative of increased risk of malignancy.
The OVA1 received FDA clearance in 2009 as an aid in assessing the risk of finding a malignancy in women presenting with an adnexal mass when independent clinical and radiological evaluation is indeterminate for malignancy. The test should not be used without these independent evaluations, nor should it be used as a screening test or for determining whether a patient should have surgery. A prospective trial involving 27 primary care and specialty sites throughout the U.S. evaluated 590 women scheduled for ovarian tumor resection and compared physicians' pre-surgery assessment only versus pre-surgery assessment with OVA1 score. The pre-surgery assessment and OVA1 score had 96% sensitivity, 35% specificity, 40% positive-predictive value (PPV), and 95% negative-predictive value (NPV); whereas the physician pre-surgery assessment arm had 75% sensitivity, 79% specificity, 62% PPV, and 88% NPV. These results demonstrated that the test is capable of identifying the majority of patients with an ovarian malignancy who are missed by preoperative physician assessment alone; however, the lower specificity of the assay might increase the number of women who are referred to a gynecologic oncologist.
Clinicians and patients need to recognize that this multi-analyte test does not replace current methods of risk assessment. Therefore, laboratory professionals should discuss appropriate test utilization with clinicians before adding the OVA1 test to the laboratory's menu of reference tests. For example, if clinical assessment or risk of malignancy index warrants referral to a gynecologic oncologist, clinicians should not order the OVA1 test because it does not add additional information. Similarly, the results of this test should not be used without an independent clinical assessment, nor is it necessary when clinical assessment favors conservative management. Most importantly, clinicians should never use the OVA1 test as a screening test for women without an adnexal mass.
The test also has several limitations that laboratory professionals should make clinicians aware of. Assay interference occurs in patients with rheumatoid factor levels of ≥250 IU/mL or triglyceride levels >4.5 g/L. Clinicians also should not order the test for women with a diagnosis of malignancy within the last 5 years. Cost is another factor that clinicians may want to consider. The test costs about $650 and is available only from Quest Diagnostics.
Using Markers with Caution
Detecting ovarian cancer in its early stages promises to improve outcomes for women. Unfortunately, accurate screening methods for early disease have eluded researchers, in part due to the complex biology of ovarian cancer.
Laboratory professionals should strongly discourage clinicians from using current biomarkers as screening tools because they lack specificity and sensitivity and their misuse might lead to unnecessary treatments for women and increased burden to the healthcare system.
The recent introduction of the biomarker HE4 and the OVA1 multi-analyte test reflect the ongoing and extensive research and validation efforts being made to improve outcomes of not only women with benign masses but also those with ovarian cancer. Researchers continue to investigate new biomarkers for screening, diagnosing, and monitoring the disease. Before these biomarkers can be introduced to clinical practice, properly designed clinical trials are needed to determine if they will become useful clinical tools. That day cannot come soon enough for the thousands of women who die each year from this silent disease and their families.
SUGGESTED READINGS
American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. Committee Opinion No. 477: The role of the obstetrician-gynecologist in the early detection of epithelial ovarian cancer. Obstet Gynecol 2011;117:742–6.
Baldwin LM, Trivers KF, Matthews B, et al. Vignette-based study of ovarian cancer screening: Do U.S. physicians report adhering to evidence-based recommendations? Ann Intern Med 2012;156:182–94.
Buys SS, Partridge E, Black A, et al. Effect of screening on ovarian cancer mortality: The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA 2011;305:2295–303.
Moore RG, Brown AK, Miller MC, et al. The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol Oncol 2008;108:402–8.
Moore RG, Miller MC, Disilvestro P, et al. Evaluation of the diagnostic accuracy of the risk of ovarian malignancy algorithm in women with a pelvic mass. Obstet Gynecol 2011;118:280–8.
Sturgeon CM, Duffy MJ, Stenman UH, et al. National Academy of Clinical Biochemistry laboratory medicine practice guidelines for use of tumor markers in testicular, prostate, colorectal, breast, and ovarian cancers. Clin Chem 2008;54:11–79.
Ueland FR, Desimone CP, Seamon LG, et al. Effectiveness of a multivariate index assay in the preoperative assessment of ovarian tumors. Obstet Gynecol 2011;117:1289–97.

Alicia Algeciras-Schimnich, PhD, is an assistant professor and co-director of the Endocrine and Automated Immunoassay Laboratories in the Department of Laboratory Medicine and Pathology at Mayo Clinic, Rochester, Minn.
Email: [email protected]
Disclosure: The author has nothing to disclose.