For decades, dental healthcare professionals have measured the buffering capacity and bacterial content of saliva to assess a person's risk of developing tooth decay (1). Today, scientific and technological advances in biochemistry, microbiology, and immunology are leading to the discovery of new biomarkers in saliva that can be used to detect systemic illnesses such as ischemic heart disease and heart failure (2–4) and cancer (5). This growing recognition of the association between an individual's oral and overall health has led to renewed interest in using saliva as a diagnostic fluid.
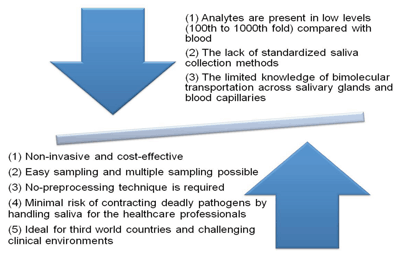
In fact, human saliva offers several benefits compared to traditional blood-based biochemical analyses. Saliva collection is non-invasive and therefore stress-free for patients. For the person collecting the sample, saliva also poses minimal risk for contracting infectious diseases such as HPV, HCV, and HIV. Finally, saliva is an ideal biofluid for developing countries due to the low cost of collecting and processing samples (Figure 1).
This article describes the current state of knowledge for saliva diagnostics and the obstacles that researchers, manufacturers, and laboratorians will need to overcome before saliva is widely accepted as a reliable diagnostic fluid.
Figure 1
The Advantages and Disadvantages of Saliva as a Diagnostic Fluid
A Saliva Primer
Three major glands—the parotid, submandibular, and sublingual—and about 400 minor glands located within the oral cavity produce saliva. A healthy adult produces an average of 500–1,500 mL saliva/day at a rate of approximately 0.5 mL/min. Human saliva has a multitude of functions within the oral cavity, including: maintaining homeostasis; promoting wound healing; lubricating the oral cavity; facilitating mineralization of dental surfaces; digesting carbohydrates by salivary α-amylase; digesting lipids via salivary lipase; and facilitating chewing, speaking, swallowing, and taste perception (6, 7). While the human mouth provides good growth conditions for many microorganisms, the anti-microbial properties of saliva also help maintain oral hygiene by clearing and inhibiting growth of microorganisms (8).
Human saliva harbors a repertoire of proteins, lipids, RNA, DNA, and some 700 microbial species. To move toward less invasive clinical management of patients, researchers have been pursuing saliva as a biofluid for early disease detection and prognosis, risk stratification, and monitoring treatment response. In fact, saliva has already been used as a biological fluid for diagnosis and prognosis of oral, head, and neck cancers, periodontal diseases, diabetes, and autoimmune disorders.
Researchers also have found other diagnostic uses for saliva. One research team identified biomarkers in saliva for detecting early-stage pancreatic cancer (9), and another measured soluble c-erbB-2Her2/neu levels in saliva collected from breast cancer patients and concluded that c-erbB may be useful in detecting and monitoring recurrence of this disease (10).
Current research also is producing valuable datasets for further analysis. Salivaomics is an open-access database that contains salivaomics-based studies and includes information on the biology, diagnostic potential, pharmacogenomics, and pharmacoproteomics of saliva.
Saliva Proteome
Human saliva is a plasma ultra filtrate and contains proteins either synthesized in situ in the salivary glands or derived from blood. It contains biomarkers derived from serum, gingival crevicular fluid, and mucosal transudate. To date, researchers have identified 2,340 proteins in the salivary proteome, of which 20–30% are also found in blood (11), an encouraging indicator for the clinical utility of saliva as a diagnostic fluid.
In contrast to the plasma proteome, in which 99% of the total protein content is contributed by 22 highly abundant proteins (8), the 20 most abundant proteins in human whole saliva (WS) constitute only 40% of the protein content (12). This composition suggests that detecting biomolecules of clinical sensitivity and specificity in saliva should be feasible and easier than in blood. Unlike the plasma proteome, however, the WS proteome is highly susceptible to a variety of physiological and biochemical processes, such as salivary protein modifications occurring in the mouth that are catalyzed by host and bacterial derived enzymes. Such modifications also could present unique challenges for clinical saliva proteomics.
The dynamic range of proteins in saliva is another challenge. For instance, α-amylase, an abundant protein in saliva, is present at mg/mL concentrations, while the IL-6 and IL-8 cytokines of potential clinical relevance are present at concentrations of only pg/mL. This disparity highlights the importance of developing tools and strategies for discerning low abundance proteins with clinical relevance in saliva.
How molecules are transported from blood into saliva may also be important for successful use of saliva as a diagnostic fluid. Lipophilic molecules such as steroid hormones passively diffuse into saliva, while water and electrolytes pass through the pores of acinar cells. Various peptides in blood move through protein channels, and large proteins are transported via pinocytosis (4).
Commercially Available Saliva Tests
Two U.S. companies were early pioneers of oral diagnostics: Epitope, Inc. and Saliva Diagnostic Systems, Inc. They both commercialized saliva collection devices in the early 1990s, and in 1996 the Food and Drug Administration (FDA) approved Epitope's Orasure HIV test, the first test that used oral fluid to test for an infectious disease.
More recently, FDA approved the first over-the-counter salivary HIV test that allows people to test themselves in the privacy of their homes for the HIV virus. The OraQuick HIV test, which takes only 15 minutes from start to finish, detects the presence of HIV antibodies in saliva via mouth swab.
Several companies outside the U.S. have commercial tests to detect drugs-of-abuse in a spit sample, including Cozart Biosciences, Securetec, and Mavand. Some of these companies send their kits via regular mail to customers, allowing individuals to collect their own saliva either in a cup or with a swab and then send the sample to a laboratory for analysis.
Other tests target DNA in saliva. Canada-based DNA Genotek was the first company to commercialize a broad range of saliva collection tools for genotyping based on the polymerase chain reaction (PCR), microarrays, and sequencing. Oral DNA Labs, a subsidiary of Quest Diagnostics, also offers two salivary tests in the U.S. in its CLIA-approved testing facility. My PerioPath is a DNA test that determines the risk of periodontal infections by detecting bacterial pathogens in saliva. OraRisk HPV is a salivary test that determines an individual's risk of developing HPV-related oral cancers. It identifies various HPV genotypes, including HPV 8, 11, 16, and 18.
Emerging Clinical Applications
Other applications of salivary diagnostics are emerging, including for the detection of cardiovascular disease (2–4) and head and neck cancer (5).
Our group and others have demonstrated that salivary C-reactive protein (CRP) levels can be used as a biomarker to differentiate patients with ischemic heart disease from healthy controls (2, 3) (Figure 2). Work by Denver et al. also has demonstrated that salivary endothelin concentrations (13) and salivary natriuretic peptide levels (4) are useful as biomarkers to assess heart failure.
Figure 2
Salivary CRP Levels
A. Salivary CRP levels in healthy volunteers (n = 55) and in patientswith ischemic heart disease (n = 28).
B. Correlation of salivary to plasma CRP levels.
In addition, we tested commercially available saliva collection devices (Figure 3) to determine their efficacy for detecting salivary proteins with varying molecular weights, such as CRP, myoglobin, and IgE, in healthy controls (14) (Table 1). Our data demonstrated significant differences in analyte levels based on the collection device.
|
Table 1
Efficacy of Saliva Collection Methods
|
| Analyte |
Collection Device |
Drool cup
Median/IQR |
Salimetrics
oral swab
Median/IQR |
Salivette
cotton swab
Median/IQR |
Salivette
synthetic swab
Median/IQR |
| CRP (pg/mL) |
28/9–51 |
9/5–30* |
19/7–46 |
14/5–44 |
| IgE (pg/mL) |
72/38–202 |
72/35–123 |
43/30–153 |
37/30–164* |
Myoglobin
(pg/mL) |
98/31–140 |
23/2–54* |
23/12–52** |
59/34–136 |
| The table summarizes data for the median and interquartile range (IQR) levels for three analytes collected by four different methods. We observed significant differences (*p<0.05) for both CRP and myoglobin levels using the drool cup versus the Salimetrics collection device, as well as significant differences (**p<0.01) for myoglobin using the drool cup versus the Salivette cotton swab. For IgE, the levels collected in the drool cup versus the Salivette synthetic devices were correlated more closely (*p<0.05) (14). |
Early detection of head and neck squamous cell carcinoma (HNSCC), cancers that occur in the nasal passages, sinuses, mouth, larynx, and pharynx, is another focus of salivary diagnostics. Tobacco use is a major risk factor for this type of cancer, and 30–50% of HNSCCs are a direct result of HPV 16, and to a lesser extent HPV 18, infections. DNA methylation in cells is an early event that occurs during tumor initiation, and we have found methylation of three tumor suppressor genes (DAPK1, RASSF1A and p16) in saliva collected from HNSCC patients that is not present in healthy controls (5).
Figure 3
Saliva Collection Devices
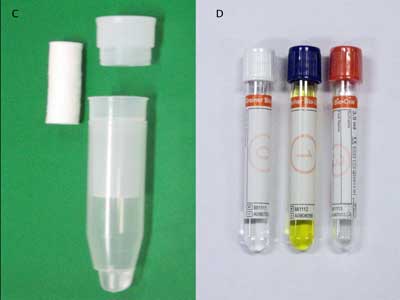
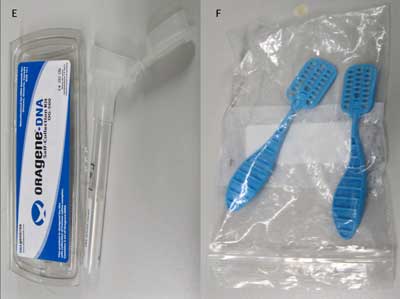 Shown here is the wide variety of commercially available saliva collection devices in use today: drool collected in a sterile specimen container (A); Salimetrics oral swab (B); Salivette cotton and synthetic device (C); Greiner Bio-One saliva collection system (D); OriGene DNA collection device (E); and DNASal collection device (F).
Shown here is the wide variety of commercially available saliva collection devices in use today: drool collected in a sterile specimen container (A); Salimetrics oral swab (B); Salivette cotton and synthetic device (C); Greiner Bio-One saliva collection system (D); OriGene DNA collection device (E); and DNASal collection device (F).
Roadblocks to Advancement
Expansion of salivary diagnostics is hampered by several factors. First, analytes in saliva are usually present at only 0.1–0.001 of the levels found in blood; therefore, very sensitive detection technology is required to develop effective tests. Another impediment is the lack of information about baseline levels or reference ranges of molecules in saliva within a healthy control population. This information is crucial to discriminating disease-specific changes.
To be clinically useful, there also must be reliable correlations between levels of the target substance in saliva and in blood or plasma. For example, we know that salivary diagnostics are not well suited to measuring glucose levels because blood and salivary levels of this analyte are poorly correlated. This could be the case for other analytes as well.
Another complication is that contamination of saliva samples with even a small amount of blood will lead to a false-positive result. In fact, bleeding after brushing or flossing occurs quite frequently and could contribute to high false-positive rates for salivary tests. Research also is needed on how levels of molecules vary diurnally. For example, we know that salivary growth hormone levels are higher in the morning than during the day, which could also be the case for other biomarkers.
The lack of standardized saliva collection methods also makes the widespread adoption of salivary diagnostics more challenging. In one study, researchers reported that the OraSure saliva collection device detects hepatitis C virus with greater sensitivity than the Salivette device (15). Such differences may be related to the collection device itself.
What Does the Future Hold?
As our knowledge of the biomolecules present in saliva grows, the potential applications for oral and systemic disease diagnosis will expand. While the scientific link between salivary biomarkers and oral diseases is clear, more studies are needed to delineate the mechanisms by which saliva reflects other systemic diseases. Furthermore, before saliva can become widely recognized as a reliable diagnostic fluid, we need to more fully understand a number of important variables.
First, we need to define the normal biological variability of biomolecules in saliva, such as diurnal rhythms, inter- and intra-subject variation, and age and gender effects. The influence of diet, medication, smoking, alcohol, and physical activity status may also influence levels of biomolecules in saliva. From an analytical standpoint, methodological variations caused by saliva sampling, handling, and storage conditions will need to be defined, as well as methodological variations due to the analytical techniques used. Since the salivary proteome is sensitive to both extrinsic and intrinsic factors, analyte reference ranges in saliva will need to be carefully documented.
Salivary diagnostics has enormous potential for the future, but we need to lay a solid scientific foundation in the present in order to realize that potential. Non-invasive tests for detecting breast cancer, viral, and bacterial diseases, cardiovascular and metabolic diseases, and general nutritional deficiencies could make a tremendous impact on global health.
The key parties responsible for translating salivary research from a laboratory setting to clinical practice, including scientists, regulatory agencies, and third parties such as insurance companies, will need to work together to determine how new saliva diagnostics are adopted by the health care community. But undoubtedly, a saliva swab test in the privacy of one's home or at the general practitioner's office will become a reality for diagnosing systemic diseases.
REFERENCES
- Lawrence HP. Salivary markers of systemic disease: noninvasive diagnosis of disease and monitoring of general health. J Can Dent Assoc 2002;68:170–4.
- Christodoulides N, Floriano PN, Miller CS, et al. Lab-on-a-chip methods for point-of-care measurements of salivary biomarkers of periodontitis. Ann N Y Acad Sci 2007;1098:411–28.
- Punyadeera C, Dimeski G, Kostner K, et al. One-step homogeneous C-reactive protein assay for saliva. J Immunol Methods 2011;373:19–25.
- Yang Foo JY, Wan Y, Kostner K, et al. NT-ProBNP levels in saliva and its clinical relevance to heart failure. [Epub] PLoS One October 31, 2012 as doi:10.1371/journal.pone.0048452.
- Ovchinnikov DA, Cooper MA, Pandit P, et al. Tumour-suppressor gene promoter hypermethylation in saliva of head and neck cancer patients. Transl Oncol 2012;5:321–6.
- Pfaffe T, Cooper-White J, Beyerlein P, et al. Diagnostic potential of saliva: Current state and future applications. Clin Chem 2011;57:675–87.
- Mohamed R, Campbell JL, Cooper-White J, et al. The impact of saliva collection and processing methods on CRP, IgE, and Myoglobin immunoassays. CTM 2012;1:19.
- Schulz BL, Cooper-White J, Punyadeera CK. Saliva proteome research: Current status and future outlook. [Epub ahead of print] Crit Rev Biotechnol May 21, 2012.
- UCLA Newsroom. Researchers find biomarkers in saliva for detection of early-stage pancreatic cancer. Available at the UCLA Newsroon online. (Accessed August 2012).
- Streckfus CF, Mayorga-Wark O, Arreola D, et al. Breast cancer related proteins are present in saliva and are modulated secondary to ductal carcinoma in situ of the breast. Cancer Invest 2008;26:159–67.
- Bandhakavi S, Stone MD, Onsongo G, et al. A dynamic range compression and three-dimensional peptide fractionation analysis platform expands proteome coverage and the diagnostic potential of whole saliva. J Proteome Res 2009;8:5590–600.
- Loo JA, Yan W, Ramachandran P, et al. Comparative human salivary and plasma proteomes. J Dent Res 2010;89:1016–23.
- Denver R, Tzanidis A, Martin P, et al. Salivary endothelin concentrations in the assessment of chronic heart failure. Lancet 2000;355:468–9.
- Topkas E, Keith P, Dimeski G, et al. Evaluation of saliva collection devices for the analysis of proteins. Clin Chim Acta 2012;413:1066–70.
- Judd A, Parry J, Hickman M, et al. Evaluation of a modified commercial assay in detecting antibody to hepatitis C virus in oral fluids and dried blood spots. J Med Virol 2003;71:49–55.
 Chamindie Punyadeera, PhD, is the lead research scientist in the Saliva Translational Research Group, Tissue Engineering and Microfluidics Laboratory, Australian Institute for Bioengineering and Nanotechnology at the University of Queensland in Queensland, Australia.
Chamindie Punyadeera, PhD, is the lead research scientist in the Saliva Translational Research Group, Tissue Engineering and Microfluidics Laboratory, Australian Institute for Bioengineering and Nanotechnology at the University of Queensland in Queensland, Australia.
Email: [email protected]
Disclosure: The author has nothing to disclose.