Few programs in modern laboratory medicine can boast so much success at so little relative cost as newborn screening. This year marks the 50th anniversary of newborn screening in the U.S., a program that originally looked for just one disorder, phenylketonuria (PKU). The filter paper cards developed to capture small blood samples from infant heel pricks still bear the name of Robert Guthrie, MD, PhD, who invented the bacterial inhibition assay for PKU and championed widespread screening for the condition beginning in 1963. Today, state public health labs screen some 4 million babies in the U.S. every year for more than 50 disorders, and approximately one in every 300 newborns has a condition that can be detected through screening.
However, even as public health laboratories are grappling with the promise of new technology such as tandem mass spectrometry and next generation sequencing, both laboratorians and public health advocates are alarmed that Congress may allow newborn screening legislation to expire later this year. Seeking to continue the success of newborn screening in the U.S., AACC has joined a coalition led by the March of Dimes in support of the Newborn Screening Saves Lives Reauthorization Act. The act would fully guarantee funding for newborn screening research, quality management, and follow-up as labs work toward adding new tests and prepare for the imminent genomic era.
Reauthorizing the act, first signed into law by President Bush in 2008, is essential for maintaining large strides states have made over the past 5 years, according to March of Dimes' senior vice president of public policy and government affairs, Cindy Pellegrini. "Passage of this legislation is essential to the continued functioning and effectiveness of all the newborn screening programs that it renews," Pellegrini said. "With this bill, we'll examine the programs that underpin the state newborn screening enterprise and ensure that they still are structured properly, that they serve public needs appropriately, and that we can rely on them to continue to be successful in the future."
Rapid Progress as States Adopt More Tests
The remarkable progress in newborn screening owes much not only to technological innovations, but also to advocacy campaigns led by nonprofit activists that culminated in the passage of the original legislation in 2008. Until it expired in April, this legislation had secured millions of dollars for research at the National Institutes of Health (NIH) and lab quality and education programs at the Centers for Disease Control and Prevention (CDC). The act also funded a team of experts under the Health Resources and Services Administration (HRSA) known as the Secretary's Advisory Committee on Heritable Disorders in Newborns and Children. This committee, which advises the Secretary of Health and Human Services (HHS) about the most appropriate application of universal newborn screening tests, technologies, policies, guidelines, and standards, developed the list of 31 core conditions in its uniform screening panel that all states are encouraged to adopt.
Although state legislatures still decide which disorders their screening panels will cover, the advisory committee's rigorous evidence reviews and recommendations carry a lot of weight, and have helped push through many more tests into screening programs around the country. For example, at the time of the law's passage in 2008, some states mandated screening for as few as nine conditions while others mandated more than 40. This meant that about one in five babies with one of these conditions potentially went unscreened (See interview with the bill's sponsor, below).
Congresswoman Roybal-Allard Champions Newborn Screening

Rep. Lucille Roybal-Allard (D-Calif.), along with Rep. Mike Simpson (R-Idaho), this year introduced the Newborn Screening Saves Lives Reauthorization Act. This legislation reauthorizes federal programs in the Health Resources and Services Administration, the National Institutes of Health, and the Centers for Disease Control and Prevention that assist states in improving and expanding their newborn screening programs.
How did newborn screening become an issue you are passionate about?
It was after the birth of my first child, when a nurse asked me if I wanted my daughter to have only the required tests or all the tests for the conditions that would ensure a healthy baby, that I learned about the importance of newborn screening. Naturally, like all mothers, I wanted my baby to be tested for whatever would ensure her healthiest future and I was fortunate to be able to pay for the additional costs. Later, as a legislator I became aware of the fact that there was not a national standard for screening newborn babies and that the number and quality of newborn screening tests varied greatly from state to state.
This is why I sponsored the original legislation. In 2007, only 10 states and the District of Columbia required infants to be screened for all the recommended disorders. Today, 44 states and the District of Columbia require screening of at least 29 of the 31 core treatable conditions. Much of this progress has been the direct result of passage of the Newborn Screening Saves Lives Act.
How can professional organizations like AACC help support the passage of this legislation?
Professional organizations can play a critical role in helping to educate the public and legislators around the country about policy issues that impact public health issues. This year marks the 50th anniversary of newborn screening in the United States and it is a great opportunity for AACC to help educate members of congress about the importance of newborn screening. This could be tremendously helpful in getting members to cosponsor the House Newborn Screening Saves Lives Reauthorization bill and to ensure swift passage of this important legislation.
How could this bill affect your constituents in the 40th district of California?
Newborn screening is important for all babies born in the United States—including those born in the 40th Congressional District of California—because it helps prevent babies and their families from unnecessarily experiencing the pain and suffering that occurs when a treatable condition is missed because a test was not performed immediately after birth.
The Newborn Screening Saves Lives Act helps ensure the most current and accurate science is reflected in national newborn screening recommendations and in the information made available to providers and consumers. Since passage of the original legislation, the Secretary's Advisory Committee for Heritable Disorders, which was authorized by the bill, suggested the addition of two new screenings to the recommended screening panel. The most recent was Critical Congenital Heart Disease (CCHD)—a common birth defect, affecting approximately eight of every 1,000 newborn babies. In California, an estimated 30 babies die each year from undiagnosed CCHD. These deaths could be prevented if CCHD is detected and treated earlier. I am proud to say that on July 1, California added CCHD to its state newborn screening program. Today, California screens for all 31 conditions recommended by the secretary of the U.S. Department of Health and Human Services.
What is your message to other members of Congress?
I tell my colleagues in Congress that newborn screening is a public health success story. For 50 years, newborn screening has saved countless lives. For the more than 12,000 babies who test positive for one of these conditions each year, rapid identification and treatment make the difference between health and disability—or even life and death. For families, this early intervention can mean avoiding the emotional and costly diagnostic journey to determine why their newborn is failing to thrive. Supporting newborn screening and passage of the Newborn Screening Saves Lives Reauthorization Act makes sense—because no baby should die or suffer the devastating health consequences of a condition that could have been treated or prevented if identified through newborn screening.
Now, all states screen for at least 29 of the 31 core disorders recommended by the advisory committee, according to the March of Dimes, and many bills are pending in state legislatures to add more (See Box, below).
The success of newborn screening depends on a synergy between the federal government and nonprofit groups that advocate for change at the state level, according to R. Rodney Howell, MD, chairman emeritus and professor of pediatrics at the Miller School of Medicine at the University of Miami. "I think it's very exciting to see the disparity among states almost erased over the last few years, and I think it happened because we had this national body making recommendations," he said. "The nation benefited from a very distinguished group of physicians and scientists examining the evidence and saying, here is what you should screen for. Then the advocates at the state level could run with it and make sure it happened." A member of the Hussman Institute for Human Genomics, Howell also served as the founding chair of the federal advisory committee.
The reauthorization bill is also critical so that scientists at NIH and CDC don't lose momentum, noted Peter Kyriacopoulos, senior director of public policy at the Association of Public Health Laboratories (APHL). Under the reauthorization act, which APHL also supports, these agencies will share approximately $25 million in funding between 2014–2018. "One of the most important things the original bill did in 2008 was to authorize the CDC quality assurance program. Before that, funding was never assured on an annual basis, so CDC had to scramble to secure funding every year," Kyriacopoulos said.
Newborn screening is also a smart, cost-effective investment of public dollars, Kyriacopoulos added. For example, a study found that the cost to Medicaid of treating a baby with severe combined immunodeficiency (SCID) can exceed $2 million. However, if screened, diagnosed, and treated early, a bone marrow transplant that costs approximately $100,000 can cure the baby (J Allergy Clin Immunol 2012;129:597–604).
If labs are to successfully adopt new technologies and add new tests to their newborn screening menus, NIH research needs to continue uninterrupted, Howell emphasized. "As we've moved along through the tandem mass spectrometry era and now toward the genomic era, virtually all of the discoveries we're using in newborn screening have evolved out of NIH-funded research," he said.
The act may also affect crucial CDC programs that support newborn screening, most notably proficiency testing programs that would not otherwise be available, noted Mei Baker, MD, co-director of the newborn screening laboratory at the Wisconsin State Laboratory of Hygiene (WSLH) in Madison. "The quarterly proficiency testing samples provided by CDC have become an essential part of newborn screening, and I know our laboratory could not operate without it," Baker said. "For many of these assays, CDC is the only source for proficiency testing."
Status of Newborn Screening
The federal government’s Advisory Committee on Heritable Disorders in Newborns and Children reviews evidence and proposes conditions for the U.S.-recommended uniform screening panel. States pass their own laws about which conditions state public health labs must provide screening for, but the federal advisory committee’s screening panel serves as the gold standard against which advocates measure the progress of newborn screening.
The current panel includes 31 conditions. No state yet screens for all of these, but many screen for all but one. Many also include dozens of other conditions not yet added to the core panel. Since 2009, all states have screened for at least 29 of the 31 core conditions.
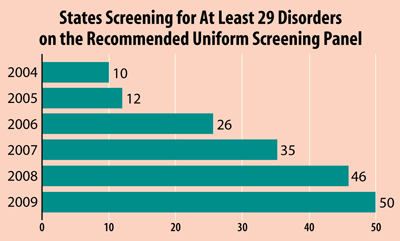
Source: Data reported from National Newborn Screening and Genetics Resource Center
States Adopt New Conditions, New Technology
Since the Newborn Screening Saves Lives act was authorized, the advisory committee has reviewed evidence for 12 nominated conditions and recommended three additions for the uniform screening panel: SCID, critical congenital heart disease (CCHD), and glycogen storage disease type II (Pompe), the latter of which HHS Secretary Kathleen Sebelius is expected to finalize approval for before the end of the year.
Pompe disease is an inherited lysosomal storage disorder in which cells cannot break down glycogen due to a deficiency of the lysosomal acid alpha-glucosidase enzyme. It exemplifies the recent technological leaps made in newborn screening, where labs increasingly depend on tandem mass spectrometry (MS/MS) to identify multiple conditions at once.
In fact, MS/MS has been instrumental in allowing labs to add new tests to their screening menu. After several public health labs began using MS/MS in the late-1990s, HRSA commissioned a report in 2005 from the American College of Medical Genetics (ACMG) that supported MS/MS programs as a way to expand screening and include more conditions, spurring MS/MS adoption. Now most state labs are using MS/MS, which allows screening for up to 40 conditions with a single assay and dried blood spot sample, according to APHL.
Molecular testing employing polymerase chain reaction (PCR) has also made its mark on newborn screening, for example with SCID. If undiagnosed, babies with SCID eventually develop severe life-threatening infections with a 100% mortality rate, usually within the first year of life. With prompt diagnosis and treatment before the infections begin, however, SCID is curable when treated by hematopoietic stem cell transplantation.
At WSLH in 2007, Baker followed up on NIH-funded research that showed a proof-of-concept screening test for SCID. Within a year, Baker developed, validated, and led implementation of a PCR assay, making the Wisconsin program the first in the world to screen for SCID routinely. The assay Baker developed is also the first molecular assay used as a primary screening test in newborns.
To develop the test, Baker collaborated with researchers at the Children's Hospital of Wisconsin and Medical College of Wisconsin on a real-time PCR molecular assay that detects the absence of T cell receptor excision circles (TRECs) using dried bloodspots. TRECs are small pieces of DNA generated in T cells as they mature. TRECs represent newly released T cells from the thymus, Baker explained. For this reason, TREC assays were first used to monitor patients with HIV during highly active antiretroviral therapy.
While initial funding came from the Jeffrey Modell Foundation, a primary immunodeficiency-focused nonprofit, as well as Children's Hospital of Wisconsin and WSLH, Baker noted that CDC support was critical for Wisconsin's three year SCID pilot program. CDC also helped other states implement SCID screening. This funding continues to allow Baker to train laboratorians in other states to screen for SCID and perform other molecular tests. Today, 11 other states routinely screen for SCID.
Going forward, molecular assays will not only allow testing for more conditions, but also provide opportunities to improve current testing schemes, Baker said. For example, cystic fibrosis (CF) screening currently relies on an immunoreactive trypsinogen (IRT) assay. Most newborn screening programs follow up elevated IRT with a DNA test for mutations in the CFTR gene from the same bloodspot. Babies with two mutations are likely to have CF. However, labs test for a fairly limited panel of mutations, and with some 1,800 total mutations reported for this gene, parents are often asked to bring babies back to the hospital for sweat chloride testing to confirm a diagnosis or rule out CF when only one mutation is identified. "If we had the capacity to include more disease-causing mutations in our molecular tests, we might rethink how we report out screening results, and as a consequence, could refer fewer patients for sweat chloride tests," Baker said.
Looking Toward the Genomic Era
Currently, whole genome sequencing is too expensive, and the data analysis it requires too onerous, for the technology to become a routine part of newborn screening. In the meantime, sequencing will evolve as follow-up testing for certain conditions after babies test positive using traditional methods, experts said.
Many conditions for which labs screen have variable courses of disease. Looking deeper into the genome to identify the likely progression of an illness should be the next line of inquiry for researchers, Howell emphasized. He cited sickle cell disease as one example. "When we find a baby who is positive for sickle cell disease today, we don't know whether or not that baby is going to be somebody who has a lot of problems," Howell said. "Some grow up into adulthood and, while they have complications, they do pretty well. On the other hand, some have really terrible problems right off—sickle cell crises, anemia, and profound pain—such that we would consider hydroxyurea treatment or even a bone marrow transplant. So the severity is wide ranging, even for a disease that we know so much about."
Krabbe, also known as globoid cell leukodystrophy, is another example. Many babies with Krabbe rapidly move in a downward spiral toward an early, painful death. Yet others who have similar enzyme activity detected by newborn screening live longer, healthier lives, Howell noted.
As whole genome sequencing becomes more integrated into newborn screening, laboratorians and policymakers will need to consider how far newborn screening should go, Baker said. "Right now, newborn screening's purpose is very well defined—to detect disease and get early treatment. But with whole genome sequencing, you get information about pharmacogenomics and about risks for disorders or health problems down the road. This goes well beyond even the discussion about screening versus a diagnostic test," Baker commented. "We will need all these resources at CDC, NIH, and HRSA and states working together as we move toward the inevitable with genomic information."
Make Your Voice Heard
Show your support for newborn screening legislation using AACC’s Advocacy Network. You can quickly compose and email a message to your elected representatives in Congress by simply entering your zip code. Access the AACC Advocacy Network online.