
Infertility is a common clinical problem affecting an estimated 15% of couples worldwide. According to the 2006 National Survey of Family Growth, approximately 7.4 million women age 15–44 in the U.S. reported receiving any type of infertility service. But with current technology, women who undergo infertility treatments enjoy much improved success rates.
Infertility treatments can consist of an extensive clinical evaluation, including multiple laboratory and imaging studies. Laboratory testing for female hormones plays a large part in both the work up for infertility and monitoring during treatment. This review will discuss the laboratory tests commonly used for these purposes, including tests of importance during in vitro fertilization (IVF) cycles.
Follicle Stimulating Hormone
Human females are born with a finite amount of oocytes. Oocyte loss via apoptosis occurs throughout the lifespan, even in prepubertal females. For reproductive age females, cyclic cohorts of oocytes begin development in any given menstrual cycle, but typically only one becomes fully mature and is ovulated. Although there is significant individual variation, in general, oocyte quantity and quality begin to decline rapidly around age 37 and continue to fall until the oocyte supply is exhausted in menopause.
Determining a woman’s relative ovarian reserve, or quantity and quality of oocytes, is a critical early part of the initial infertility evaluation. Although testing protocols vary, most IVF centers use a combination of laboratory studies and ovarian imaging via ultrasound to determine ovarian reserve and help guide fertility therapy.
High serum follicle stimulating hormone (FSH) concentrations in the early part of the menstrual cycle are a well-known predictor of reproductive aging; therefore, clinicians for many years have used FSH testing as a component of ovarian reserve testing. To determine ovarian reserve, clinicians measure a woman’s FSH value at its nadir early in the follicular phase, cycle days 2–3. While FSH is strongly associated with reproductive age, it also varies during a woman’s lifetime, as well as throughout a typical menstrual cycle. Given this large amount of variation, many studies have demonstrated that maternal age, rather than FSH value alone, may be more predictive of IVF success (1). FSH values >10 IU/L seem to have high specificity for predicting poor response to ovarian stimulation, but the sensitivity at this value is low unless FSH is at a high threshold value.
Very high levels of FSH, however, seem to be predictive of poor pregnancy outcome. In one recent study, FSH levels >18 IU/L resulted in no live births (2). Consequently, some fertility programs have FSH thresholds above which women are not offered IVF services due to the low probability of success.
Estradiol
Estradiol (E2) is a steroid hormone secreted into circulation by granulosa cells of developing ovarian follicles. Clinicians also commonly measure E2 levels in women as part of ovarian reserve testing. Levels of this hormone in reproductive age women fluctuate from 10–300 pg/mL, depending on timing of the menstrual cycle (3). For ovarian reserve testing, clinicians typically assess E2 levels at their nadir early in the menstrual cycle, at days 2 or 3.
Assessing levels of these hormones at a consistent time point in the menstrual cycle allows clinicians to make meaningful interpretations of the values. For example, a high basal FSH or E2 level suggests impaired oocyte development early in the menstrual cycle—a worrisome sign of reproductive aging and/or poor ovarian reserve. In addition, because estradiol exerts negative feedback on FSH secretion from the pituitary gland, high E2 (>100 pg/mL) can inhibit FSH secretion, resulting in an artificially low FSH value.
Despite being a simple, inexpensive, and effective screening tool, basal E2 levels alone have a low predictive value for IVF outcome. In a systematic review of 3,352 patients being treated for IVF, researchers actually found conflicting results (4). In some studies, lower IVF success rates were reported for both low and high E2 levels, whereas others found that baseline E2 levels have no effect on IVF success. In yet another study, researchers discovered that a basal estradiol level >75 pg/mL was associated with poor IVF outcomes and pregnancy rates (3). Given these inconsistencies, the best use of E2 levels in combination with baseline FSH values has been in determining the appropriate medication doses to start an IVF cycle or for deciding to forego treatment altogether when the probability of success is low.
In addition to baseline ovarian function, clinicians frequently evaluate women’s E2 levels in a controlled-ovarian-hyperstimulation (COH) setting to monitor follicular development. As follicles develop, E2 levels steadily increase. During a spontaneous cycle, E2 levels typically peak between 250–300 pg/mL as ovulation approaches. In contrast, when superovulation—release of more than one mature oocyte—is the goal, women in a COH cycle have significantly higher E2 values (Figure 1).
|
Estradiol and Follicular Diameter in IVF by Day of Stimulation
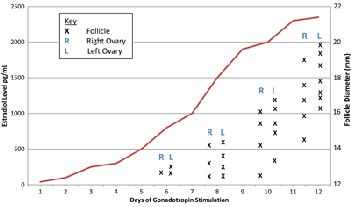
Both estradiol and average follicular diameter increase throughout a typical cycle.
|
In both COH and IVF cycles, clinicians use E2 levels to determine the risk of ovarian hyperstimulation syndrome (OHSS), a self-limited but occasionally severe response to ovarian hyperstimulation. In a retrospective analysis of IVF patients, researchers defined OHSS risk groups according to estradiol levels (5). In 637 IVF cycles, no patients developed OHSS with peak E2 levels <3500 pg/mL, 1.5% developed hyperstimulation with peak E2 levels 3500–5999 pg/mL, and 38% developed OHSS with E2 levels >6000 pg/mL. While many factors contribute to cycle monitoring and prevention of OHSS, studies have shown that monitoring E2 levels reduces incidence of OHSS. Although the upper threshold of E2 that confers the highest risk of OHSS is inconsistent in the literature, most clinicians agree that risk of OHSS with a peak E2 level <3000 pg/mL is low risk (1).
|
Table 1
Laboratory Tests Used for Fertility Testing
|
| Laboratory Test |
Normal Values |
When to Measure |
Important Points |
| Follicle Stimulating Hormone (FSH) |
5–20 mIU/L |
Follicular phase, days 2–3 of menstrual cycle |
FSH values >10 IU/L predict poor response to ovarian stimulation (1)
FSH values >18 IU/L predictor of poor pregnancy outcome (2)
|
| Estradiol (E2) |
20–400 pg/mL |
Follicular phase, days 2–3 of menstrual cycle |
Helpful in combination with FSH to establish baseline ovarian reserve
Use with monitoring of COH. Low risk of OHSS with E2 value <3000 pg/mL
|
| Anti-Müllerian Hormone |
0.9–9.5 ng/mL |
Not cycle dependent so can be measured at any time |
Low values (0.2–0.7 ng/mL) predict poor response to COH but are not useful in predicting pregnancy (6) |
| Inhibin B |
<139 pg/mL
during the follicular phase |
Follicular phase, days 2–3 of the menstrual cycle |
Serum levels <45 pg/mL have been associated with poor response to gonadotropins (9) |
| Progesterone |
Follicular phase:
<3 ng/mL
Secretory phase: 5–30 ng/mL
|
One week prior to expected menses to assess for ovulation |
Values <3 ng/mL during the secretory phase indicate anovulation |
| Lutenizing Hormone (LH) |
5–20 mIU/mL |
Mid-follicular phase |
Studies vary in regard to clinical value of LH |
| Abbreviations: COH: controlled-ovarian-hyperstimulation; OHSS: ovarian hyperstimulation syndrome. |
Anti-Müllerian Hormone
Anti-Müllerian hormone (AMH) is a dimeric glycoprotein and a member of the transforming growth factor-beta superfamily, which, like estradiol, is secreted by granulosa cells of pre-antral and antral follicles in the ovary. Serum AMH levels reflect the overall follicular pool, and several research teams have evaluated AMH as a marker of ovarian reserve. In general, high AMH levels correlate with good ovarian reserve and vice versa. Furthermore, AMH is undetectable 3–5 days following bilateral ovariectomy. Several research groups also have demonstrated a correlation between low AMH levels and menopause (6).
In addition to its role in evaluating ovarian reserve, researchers have studied AMH as a predictor of response to gonadotropin stimulation during ovulation induction. Over the natural course of ovarian stimulation, AMH levels gradually decline. Administering FSH exogenously to women leads to an increase in follicular size and E2 levels, both of which have been implicated as regulators of AMH secretion.
Several studies also have demonstrated a strong correlation between basal AMH levels and number of oocytes retrieved during an IVF cycle, including one in which basal AMH levels were found to be 2.5 fold higher in patients who had >11 oocytes retrieved. In a meta-analysis, AMH appeared to be a better predictor of response to ovarian stimulation than patient age and day-3 FSH, E2, or inhibin B levels (7). The studies comparing AMH to antral follicle count for predicting response to ovarian stimulation found no significant difference between these two analytes (7).
Poor response to ovarian stimulation occurs in approximately 2–30% of IVF cycles. While not all IVF programs use the same definition, most classify women as poor responders based on low follicle number (<3–5) and retrieved oocytes (<3–5), as well as on cancellation of a cycle due to inadequate stimulation. La Marca et al (7) published the first study evaluating AMH as a tool for predicting poor response to controlled ovarian hyperstimulation. They found AMH had a sensitivity of 80% and specificity of 93%; however, the accuracy of AMH for predicting poor response to COH was not consistently reproducible. These findings suggest that a low response is not a good reason to exclude patients from IVF.
On the opposite end of the spectrum, AMH also may be useful for predicting OHSS. This hypothesis is based on the idea that an exaggerated response to gonadotropins may be secondary to high AMH. Two prospective trials have demonstrated that basal levels of AMH >3.5 ng/mL are associated with OHSS. Further studies are needed to determine whether stimulation treatment should be stratified based on AMH levels to avoid OHSS (7).
Inhibin B
Inhibin B is one of the beta subunits of the dimeric protein inhibin, and like AMH, it is produced by pre-antral and early antral follicles. Inhibin B values vary during a woman’s reproductive lifetime and menstrual cycle. In addition, changes in inhibin B levels that occur with reproductive aging are similar to those of FSH and AMH. As women age, FSH levels increase, and both inhibin B and AMH decrease. Decline in inhibin B level is a late marker of diminished ovarian reserve; therefore, inhibin B values cannot be used to predict ovarian failure or menopause (8). Currently, inhibin B levels are not regarded as a standard and/or reliable assessment of ovarian reserve (6).
Administering exogenous gonadotropins for controlled ovarian stimulation results in an increase in inhibin B, and studies have demonstrated that poor responders generally have lower inhibin B levels. In fact, serum levels of inhibin B <45 pg/mL have been associated with poor response to gonadotropins, as well as high rates of cycle cancellation, reduced IVF retrieval, and lower clinical pregnancy rates (9).
Progesterone
Progesterone levels are a simple measurement of ovarian function. Progesterone levels remain low during the follicular phase (<1 ng/mL), rise on the day luteinizing hormone (LH) surges (1–2 ng/mL), and increase steadily until they peak approximately 1 week after ovulation. Progesterone levels <3 ng/mL imply anovulation, except when assessed immediately after a woman ovulates or prior to menses when progesterone levels are at a physiological low. To assess for presence or absence of ovulation, clinicians typically assess a woman’s progesterone levels approximately 1 week before the expected onset of menses (6).
Clinicians often use gonadotropin-releasing hormone (GnRH) analogues to induce ovulation and suppress the pituitary, thereby preventing premature LH surge and subsequent ovulation. However, many studies describe a finding called premature luteinization that occurs in 5–30% of cases. This happens when patients’ serum progesterone levels rise higher than a defined threshold value of 0.9–1.2 ng/mL, despite their having received GnRH. Some clinicians suspect that if a woman’s serum progesterone is elevated on the day ovulation is induced, the success of the IVF cycle is adversely affected, although this has been the subject of debate for many years. A recent meta-analysis of 12 studies concluded that the probability of clinical pregnancy in IVF cycles did not differ significantly between patients with elevated progesterone and those with normal levels on the day of administering the human chorionic gonadotropin trigger (10).
Luteinizing Hormone
As stated in the previous section, inhibiting premature LH surge during ovarian stimulation for IVF has been a clinical standard for many years. Multiple studies have been conducted on the role of LH concentrations with regard to both oocyte quality and the endometrium. Two prospective studies suggested that the higher the mid-follicular endogenous LH level, the lower the probability of ongoing pregnancy achieved via IVF. In comparison, a recent meta-analysis demonstrated there was no association between low LH levels and decreased success of an ongoing pregnancy achieved via IVF (11).
Reasons to Celebrate
Today, more than 1% of all infants are born with assisted reproductive technology, a statistic expected to rise as mean maternal age continues to increase. While the workup for infertility can be extensive, laboratory tests can help determine primary diagnosis and assess ovarian reserve (Table 1, above). Most importantly, the results of these tests provide a predictive framework for managing and treating infertility.
As technology continues to improve, laboratory testing will undoubtedly become a more accurate and sensitive predictor of response to COH, making treatment of infertility safer and more effective for women. There are many reasons to celebrate now, however, as more and more infertile couples welcome babies born with these assisted technologies.
REFERENCES
- Broekmans FJ, Kwee J, Hendriks DJ, et al. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update 2006;12:685–718.
- Scott RT Jr, Elkind-Hirsch KE, Styne-Gross A, et al. The predictive value for in vitro fertility delivery rates is greatly impacted by the method used to select the threshold between normal and elevated basal follicle-stimulating hormone. Fertil Steril 2008;89:868–78.
- Buyalos RP, Daneshmand S, Brzechffa PR. Basal estradiol and follicle stimulating hormone predict fecundity in women of advanced reproductive age undergoing ovulation induction therapy. Fertil Steril 1997;68:272–7.
- Kosmas I, Kolibianakis E, Devroey P. Association of estradiol levels on the day of hCG administration and pregnancy achievement in IVF: a systematic review. Hum Reprod 2004;11:2446–53.
- Asch RH, Li HP, Balmaceda JP, et al. Severe ovarian hyperstimulation syndrome in assisted reproductive technology: definition of high-risk groups. Hum Reprod 1991;6:1391–9.
- Speroff L, Fritz MA. Clinical gynecologic endocrinology and infertility, 8th Ed. Philadelphia: Lippincott Williams and Wilkins 2011:1293–1383.
- La Marca A, Sighinolfi G, Radi D, et al. Anti-Müllerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART). Hum Reprod Update 2010;16:113–30.
- van Rooij IA, Broekmans FJ, Scheffer GJ, et al. Serum antimullerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril 2005;83:979–87.
- Seifer DB, Lambert-Messerlian G, Hogan JW, et al. Day 3 serum inhibin-B is predictive of assisted reproductive technologies outcome. Fertil Steril 1997;67:110–4.
- Venetis CA, Kolibianakis EM, Papanikolaaou E, et al. Is progesterone elevation on the day of human chorionic gonadotrophin administration associated with the probability of pregnancy in in vitro fertilitzation? A systematic review and meta-analysis. Hum Reprod Update 2007;13:343–54.
- Kolibianakis EM, Venetis CA, Tarlatzis BC. Role of the endocrine profile for the achievement of pregnancy with IVF. Reprod Biomed Online 2009;18:37–43.

Abigail Delaney, MD, is a chief resident at the University of Nebraska Medical Center in Omaha, Neb.
Email: [email protected]

Jani R. Jensen, MD, is a consultant in the Division of Reproductive Endocrinology and Infertility at Mayo Clinic in Rochester, Minn.
Email: [email protected]

Dean E. Morbeck, PhD, is IVF Laboratory director at Mayo Clinic in Rochester, Minn.
Email: [email protected]
Disclosure: The authors have nothing to disclose.