When Congress authorized the National Children's Health Study (NCS) in 2000, laboratorians immediately saw the potential for pediatric lab medicine. A longitudinal study that will follow 100,000 children across the country from before birth to age 21, NCS will collect the rarest of samples: blood from a diverse, healthy population of young children. A growing collection of such samples seemed like the ideal starting point for work on pediatric reference ranges, long a cause for concern among pediatricians and laboratorians because of the dearth of quality data, especially for children under 3 years of age.
Now, AACC is taking the lead for the lab community by funding the first pediatric reference range studies on the early samples available from NCS subjects. After working with NCS in an advisory role for 6 years, members of the AACC Pediatric Reference Range Committee (PRRC) are beginning two pilot studies that will establish age-related reference ranges for steroid hormones and amino acids.
Ultimately, the committee aims to draw in other stakeholders to continue these studies and take full advantage of what the NCS has to offer, according to chair Michael Bennett, PhD, FRCPath. "I've been a pediatric clinical chemist all of my career, and throughout that time, we've always struggled with reference ranges," he said. "One of the problems that we've always had in children's hospitals is that you don't have healthy children in the hospital, so you can't comfortably use that data. With a large cohort of healthy children from across the country, the National Children's Study offers laboratorians a unique opportunity to develop good reference ranges and also to understand better how the biomarkers that we measure in our labs reflect normal childhood development." Bennett is director of the Michael J. Palmieri Metabolic Laboratory at Children's Hospital of Philadelphia and professor of pathology and laboratory medicine at the University of Pennsylvania Perelman School of Medicine.
A New Chance to Tackle an Old Problem
Although published data exists for pediatric reference ranges, so far no study has proven complete enough or robust enough to establish widely recognized standards for lab medicine, noted Patricia Jones, PhD, the primary investigator for the AACC-funded studies. "Pediatric reference ranges are really a wide open area. Even institutions using the same instruments have different reference ranges. It's a subject that we all recognize needs a lot of work." Jones is clinical director of the chemistry and metabolic disease labs at Children's Medical Center of Dallas and professor of pathology and medical laboratory science at UT Southwestern Medical Center. When she surveyed her colleagues in other pediatric institutions on reference ranges for common analytes, like electrolytes and glucose, she discovered that the ranges varied from lab to lab (See Box, below). This weakness of reference ranges poses real consequences for patients, making interpretation difficult for some tests and potentially leading to inappropriate treatment decisions or misdiagnoses, she added.
Reference Ranges Vary by Institution
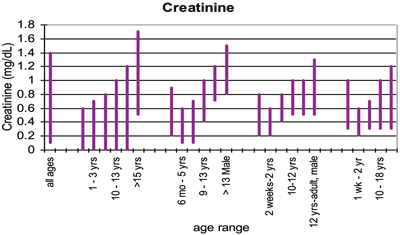 The figure shows age-based pediatric reference ranges from five pediatric institutions. In 2009, Patricia Jones, PhD, of Children's Medical Center of Dallas, informally gathered the data from several of her colleagues.
The figure shows age-based pediatric reference ranges from five pediatric institutions. In 2009, Patricia Jones, PhD, of Children's Medical Center of Dallas, informally gathered the data from several of her colleagues.
Other researchers, both in the U.S. and internationally, are addressing pediatric reference ranges, but so far cannot match the size and scope of NCS (CLN 2009;35(9). For example, Children's Health Improvement through Laboratory Diagnostics (CHILDx), sponsored by ARUP Laboratories and the University of Utah Department of Pathology, has recruited thousands of subjects to establish reference ranges for more than 100 analytes in children 7 to 17 and 45 analytes in children age 6 months to 7 years. However, all of the subjects are local to Utah and primarily Caucasian, limiting how CHILDx findings can be applied elsewhere.
The Canadian Laboratory Initiative on Pediatric Reference Intervals (CALIPER) has ambitious goals as well. The project aims to establish a comprehensive database of reference ranges for Canadian children from birth to 18 years. However, the great cost and difficulty of obtaining samples from young, healthy children has slowed progress, said Vijaylaxmi Grey, PhD, a CALIPER co-investigator and past member of the PRRC. "In CALIPER, we are collecting our own samples, which is a huge endeavor," she said. "While we have completed some pilot studies, funding has been a major issue in getting it off the ground." Only recently did CALIPER receive funding for 4 years from the Canadian Institutes of Health Research. Grey is director of pediatric clinical biochemistry in the Hamilton Regional Laboratory Program and a professor of pathology and molecular medicine at McMaster University in Hamilton, Ontario.
Grey pointed out that the most difficult samples to collect are those of neonates—the age group that now makes up the bulk of available samples from the NCS. "This is one of the major advantages of the NCS," she said. "It is still a challenge for us in CALIPER because it's such a difficult group to gain access to," (See Box, below).
Establishing Pediatric Reference Ranges
Absent a comprehensive database of data from healthy children such as the National Children's Study plans to generate, labs have had to make do with limited numbers of samples from hospitalized children.
Although standards for developing pediatric references ranges have been published by Clinical and Laboratory Standards Institute (CLSI) and other sources, they require samples from healthy children, said Patricia Jones, PhD. "One of the biggest problems with pediatric reference ranges is that according to the method for setting reference ranges, you have to use 120 samples at each age from healthy individuals, and that's impossible for a pediatric institution," she said. "As a result, labs have to set their reference ranges the best way they can, and that doesn't always involve healthy children. Should you turn around and use those reference ranges on healthy children? That's anyone's guess and there is a lot of debate about it." Jones is clinical director of the chemistry and metabolic disease labs at Children's Medical Center of Dallas and professor of pathology and medical laboratory science at UT Southwestern Medical Center.
One method many labs use is to take an entire hospitalized population and remove outliers. However, despite the availability of published statistical methods to make this work, data from sick children is still not the same as that from healthy children, commented Vijaylaxmi Grey, PhD. "I think there is a limitation to using hospitalized data," she said. "If you look at the use of banked data for adults, much more wellness testing is done. In children, however, testing is more judicious—you only test when they're ill, so the percentage of normal values in a children's hospital is much less. I don't think it should be ruled out as an approach, but I don't think it's going to be the same as what we've been able to do for adults. A reference range is by definition from a healthy, normal person." Grey is director of pediatric clinical biochemistry in the Hamilton Regional Laboratory Program and professor of pathology and molecular medicine at McMaster University in Hamilton, Ontario.
Importantly, there is a big difference between establishing reference ranges—for which experts recommend the 120 samples from healthy individuals—and verifying published reference ranges for use at a particular institution, Jones noted. For the latter purpose, the lab can use as few as 20 samples, according to the third edition of the CLSI guideline "Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory: Approved Guideline (CLSI C28-A3)."
With all the difficulties of sample collection already taken care of, and the potential for a diverse cohort spanning the nation, NCS looked like the perfect opening for the lab community to make progress on pediatric reference ranges, Bennett said. Initially, the committee focused on helping NCS collect high-quality samples, as well as recommendations for which analytes should be routinely analyzed as part of the main study protocol. With the study planning to follow subjects for 21 years, the committee advised on storage conditions of samples and traceability of assays to keep up with new technologies of the future. "Traceability is a really important issue because when we adopt methods in the future, if the numbers are different it could certainly change interpretation of the outcomes data and compromise the study," Bennett said.
Grey concurred and noted that traceability is also a major priority for CALIPER as well. "Field methods certainly must be traceable to some sort of gold standard methodology that defines the unit of measurement," she said.
A major success for the PRRC was convincing NCS to collect dried blood spots in addition to serum, Bennett emphasized. "This is very significant because, with the technologies that we're seeing, this is a medium for measuring a large number of potentially useful biomarkers," Bennett said. "We recommended they do this across the board not only because these samples store more easily, but as technology improves, even though the samples are tiny, it's possible to get quite a lot of information out of them."
Getting Started
After spending several years helping ensure that NCS would collect samples suitable for the kinds of research needed in pediatric reference ranges, in 2011 the PRRC decided it was time to take the next step and launch two supplemental methodological studies. They hope to establish a base of knowledge from working closely with NCS that can be a jumping-off point for future efforts, according to Bennett. "Basically we designed these studies as a proof of principle. We wanted to develop in-depth knowledge about how the samples and data would be accessed," he said. "Being a federally funded study, NCS has very strict rules for accessing the samples. We also chose biomarkers that we knew going forward would help impact the understanding of normal childhood development."
Jones's study focuses on reference ranges for four steroid hormones: 17-hydroxy-progesterone, androstenedione, testosterone, and aldosterone, using liquid chromatography tandem mass spectrometry (LC-MS/MS). While more will be available in the future, Jones ended up with approximately 300 samples, which NCS indicated was the most they could afford to share due to the very early nature of the NCS program. Jones selected 17-hydroxy progesterone and androstenedione because, among other reasons, the tests are frequently ordered for children with congenital adrenal hyperplasia. "We have a pretty high volume of these test that come through our lab, so we had already brought them in house for a tandem mass spectrometer," Jones said. "This is also one of those areas where reference ranges, particularly in the first months of life, are very, very important, yet we just don't have a lot of information from normal children because it's so difficult to get samples from newborns." As the primary investigator, Jones is also responsible for securing the data associated with the NCS samples, a major undertaking considering the stringent requirements of a federally funded study on children.
Jones is sharing the samples with Dennis Dietzen, PhD, who will develop reference ranges for 35 amino acids. Dietzen is medical director of the core laboratory at St. Louis Children's Hospital and an associate professor of pediatrics at the Washington University School of Medicine in St. Louis. "Amino acids are flags for many different things," Dietzen said. "From dietary intake to energy utilization, there is a wealth of information in the pattern of amino acids." Dietzen will also use LC-MS/MS, employing a technique developed in his lab that can analyze dozens of amino acids quantitatively in a 20-minute run. Prior techniques took 2 to 3 hours. Using LC-MS/MS was a strategic choice that ties into why the PRRC advised NCS to collect dried blood spots in the first place, Jones said. "Obviously, technology will likely change considerably in the next 20 years of the National Children's Study, and the nice thing about tandem mass spectrometry is that if you're starting with good, traceable standards, then the results of your assay should still make sense in 10 or 20 years," she said.
However, due to the fact that most labs use serum for these analytes, both studies will also have to examine results in comparison to other methods. "In the process of developing the assay for dried blood spots, we will develop it against a serum assay and determine how we can relate results back," Jones said.
The seed money provided by AACC will enable both studies to be complete within about a year. After that, the PRRC will encourage others in the lab community to pick up where they left off and keep these and other projects going as NCS moves forward. "A clear advantage for the NCS cohort is that we can measure the biological variation occurring within the same group of children over time," Dietzen said. "So, for example, if we track amino acids from birth all the way through age 21, we will also learn an enormous amount about normal developmental changes that happen in those amino acid profiles."
Looking for Support
With these initial studies underway, the PRRC is also ramping up efforts to build awareness and spark new collaborations with other stakeholders. For example, in vitro diagnostics manufacturers could either request NCS samples to produce pediatric reference ranges when developing new assays, or provide funding to a third party that would perform the studies. Several companies could also band together and donate reagents to a researcher to generate the data. In either case, the PRRC stands ready to offer help based on its experience with the current pilot studies.
So far, the feedback from companies has been positive, Dietzen said. "Those diagnostic manufacturers that we've talked to really believe that providing a complete interpretive picture for their assay is the right thing to do," he said. "This is just good business practice for a manufacturer. Even though pediatric samples constitute a relatively small fraction of the diagnostic market, the ability to provide accurate pediatric reference ranges can appeal to a company based on the fact that they want labs to use their equipment and reagents appropriately in every segment of the population."
The PRRC also wants to work with commercial labs that perform testing for those analytes that are already a part of NCS research. In order to mine this data to develop reference ranges, these labs would likely need to develop separate study proposals with NCS for the new research.
As a knowledge broker and coordinator, the members of the PRRC are eager to hear from those that would like learn more about working with NCS, Jones said. "My hope is to help facilitate other people doing this type of study. It's an area that needs so much work, and I'm confident that there are other people out there who want to be a part of it."