Acute pharyngitis, an inflammation of the pharynx and/or tonsils, is a common illness caused by many microorganisms. Although viruses are the main etiological agents, Streptococcus pyogenes, commonly known as group A streptococcus (GAS), is the primary bacterial cause, accounting for pharyngitis in 5%–15% of adults and 20%–30% of children worldwide (1).
GAS pharyngitis mainly affects children 3–15 years of age and can lead to suppurative and non-suppurative complications, the latter being more common in developing countries. Suppurative complications include oral or peritonsillar abscesses, cervical lymphadenitis, and rarely, septicemia.
Nonsuppurative sequelae include acute rheumatic fever (ARF) and acute glomerulonephritis. Along with GAS, beta-hemolytic group C streptococci (GCS) and group G streptococci (GGS) have also been implicated as causes of self-limiting acute pharyngitis in both children and adults (2). GCS and GGS closely resemble GAS in their shared virulence traits (hemolysins, M-protein, and extracellular enzymes) and their ability to cause a similar spectrum of infections, although GCS and GGS have not yet been associated with ARF.
Clinical Diagnosis: Whom To Test?
Accurate diagnosis and prompt antimicrobial therapy of streptococcal pharyngitis are important for preventing suppurative and nonsuppurative sequelae of the infection and reducing both duration of symptoms and transmission of the agent. But diagnosis based on clinical features alone is difficult because GAS symptoms overlap with those of viral pharyngitis.
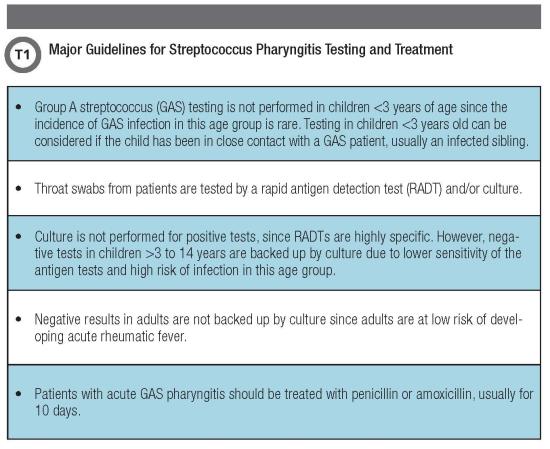
Clinicians use one of two methods, the Centor Score and McIsaac Score, for differentiating bacterial symptoms (fever, presence of tonsillar exudate, swollen anterior cervical lymph nodes, and ab-sence of cough) from typical viral features (cough, rhinorrhea, oral ulcers, conjunctivitis, and hoarseness of voice) (1,3). U.S. guidelines recommend performing timely microbiologic examination in suspected cases of pharyngitis and prescribing antibiotics to treat and prevent post-streptococcal complications (1) (Table 1, below).
A problem in diagnosing and treating true GAS infection is the presence of a carrier state, prevalent in 12%–15% of the population. A GAS carrier could be an asymptomatic patient or someone with recurrent viral infection who tests positive for GAS either by rapid antigen detection test (RADT) or throat culture.
In both cases, clinicians might administer antimicrobial therapy based on test results, which is otherwise not indicated since carriers are at low risk for transmitting the bacteria or developing post-streptococcal complications (1). Thus, it is important to be selective about the population for whom diagnostic tests are recommended to avoid identifying and treating GAS carriers.
The Role of Rapid Tests
The gold standard for detecting GAS in the pediatric population is culturing a throat swab on blood agar. However, the relatively long lag time between collecting the specimen and final microbiological diagnosis—approximately 48 hours—limits the utility of this method for routine use in outpatient settings.
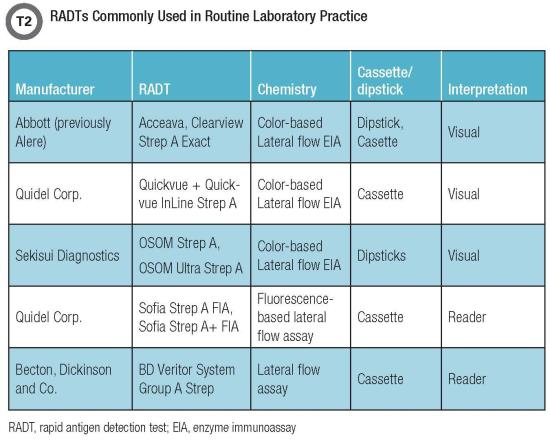
Since their inception in the early 1980s, RADTs for GAS have offered a faster and simpler alternative for outpatient settings and have aided in appropriate and early implementation of antibiotic therapy. Over time, several generations of RADTs (some of which are listed in Table 2, below) have emerged as attractive options, starting with latex agglutination, followed by color immunochromatographic and lateral flow assays (enzyme immunoassays, or EIAs) and optical immunoassays.
These assays have an in-the-device antigen extraction mechanism that extracts the carbohydrate antigen from the specimen and binds it to a highly specific anti-streptococcus antibody conjugated to reporter molecules, resulting in a signal indicating a positive test.
Although some studies have found variability in RADTs’ accuracy, especially low sensitivity, most of these assays report a high specificity, ruling out the need for a backup culture in a positive RADT result (Table 4, below). In a comprehensive review and meta-analysis of 48 studies, Lean et al. compared and evaluated the performance of commonly used RADTs (3). The summary estimate of sensitivity of RADTs among all studies was 86% (95% confidence interval [CI] 0.83 to 0.88), whereas specificity was 96% (95% CI 0.94 to 0.97) (3).
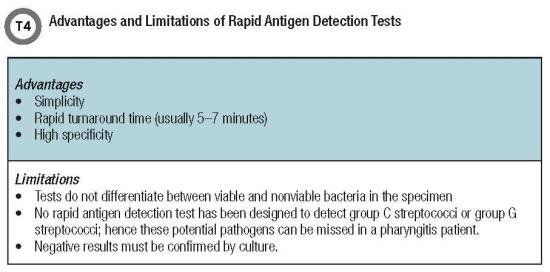
Several factors influence the performance of RADTs, such as duration of symptoms, disease se-verity, adequacy and quality of the specimen, and the test operator. In a study in which nursing staff and laboratory technicians performed the same RADT, the diagnostic performance of laboratory technicians was reported to be significantly more accurate, with a difference in sensitivity ranging from 56% to 90% between the groups (4).
This finding may be due to different levels of compliance with the method when performing the test, different levels of operator experience in performing and reading RADTs, or other unidentified reasons.
Digital immunoassays (DIAs), which incorporate an instrument to analyze the detector particles conjugated with the antigen-antibody complex, eliminated what had been a limitation of EIAs: subjective variability in visually interpreting these results. These systems are based on the principle of using either fluorescence-labeled (such as the Quidel Sofia) or other detector molecule-labeled (such as the BD Veritor) microparticles that are captured in the antigen-antibody complex.
The cassettes with the specimens can be placed either in the respective instruments for an automatically timed development (walk away mode) or on the bench top for a manually timed development of the test (read now mode). The analyzers scan, measure, and interpret the signal using specific algorithms and then report the test results to the user.
DIAs also eliminate the subjective read bias by virtue of their sensitivity in detecting weak positives that otherwise would have been missed by the unaided eye. Enhanced performance of the Quidel Sofia and BD Veritor, compared with conventional RADT, has been reported previously in studies evaluating influenza assays (5).
Similar studies comparing digital systems to traditional RADTs for analyzing S. pyogenes have yet to be reported. It remains to be seen if the claim for superior performance of DIAs also holds true for the GAS antigen test.
Molecular Assays
The persistent need for highly sensitive and rapid assays to compete against culture methods paved the way for the development of molecular assays. The first assay to be designed for this purpose was the GAS Direct probe test from Gen-Probe, now a part of Hologic. This test identified specific rRNA sequences of S. pyogenes in pharyngeal specimens by a single-stranded chemiluminescent nucleic acid probe.
GAS Direct has performed well in comparison with standard streptococcal culture methods, exhibiting a sensitivity and specificity of 94.8% and 100%, respectively (6). Results also indicated that the probe test could be used as a single primary test and that it offered the ability to report an objective result on the same day of specimen collection.
Another commercial isothermal DNA amplification assay, the Illumigene assay from Meridian Bio-science, has demonstrated excellent sensitivity (93.1%) and specificity (91.4%) for the direct detection of S. pyogenes and has emerged as an appropriate alternative to culture (7). This test relies on loop-mediated isothermal amplification (LAMP) technology with S. pyogenes-specific primers to generate results within 60 minutes on an illumipro-10 instrument.
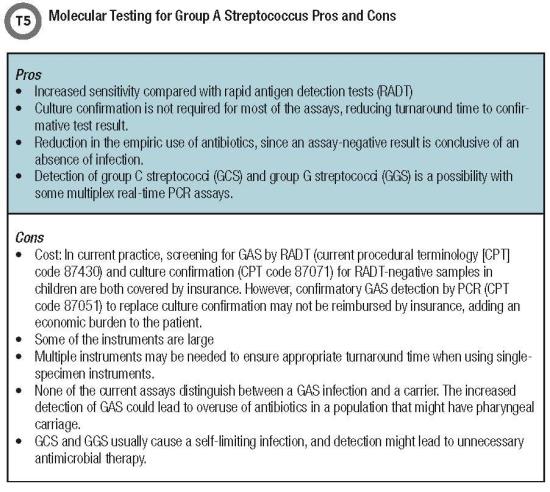
Several other nucleic acid amplification assays that are efficient, CLIA moderate complexity tests with a true sample-to-answer workflow and results within 2 hours depending on the platform, are summarized in Table 3. Apart from Food and Drug Administration-released documents, very few scientific publications have evaluated the performance of these tests for S. pyogenes detection.
More studies comparing the performance characteristics of these assays would be highly beneficial for selecting and implementing a test in clinical laboratories. It is also important to conduct carefully designed clinical studies to understand the pros and cons of GAS POC molecular testing (Table 5, below).
Is Point-of-Care Testing the Solution?
The first POC test for GAS detection on the market was the Alere i Strep A from Abbott, followed by Cobas Strep A Assay from Roche, the Xpert Xpress Strep A from Cepheid, and more recently, the modified version of Abbott’s Alere i Strep A 2 assay (11–12).
Except for the Alere i assays (isothermal DNA amplification tests), all other POC tests are real-time polymerase chain reaction platforms for GAS detection. All also exhibit high sensitivity and specifici-ty with a TAT ranging between 6 and 24 minutes and suited for front-line diagnostic testing. These POC molecular assays can replace RADTs for GAS detection and eliminate the need for culture, as reported in a comparative study with the Alere i Strep Assay (11) (Table 5, below).
The Alere i assays use the principle of the nicking enzyme amplification reaction to provide results within 8 minutes. The modifications in the amplification primers, reaction mixtures, and internal control (sequence and concentration) in the Alere i Strep A 2 version have improved the tolerance of the reaction products to the clinical matrix. The magnetic mixing of the amplification reactions also enhances the performance of this assay.
In addition, an elimination of the initial step of warming the reagents has reduced TAT from 8 minutes to 6 minutes, making it the fastest platform for GAS detection.
Two Sides of the Same Coin
In a 2017 study, patients complaining of pharyngitis who received GAS ¬testing were evaluated for viral symptoms (13). Results showed that almost two-thirds of patients in the study had viral features, indicating unnecessary testing for many patients unlikely to have GAS pharyngitis (13). Along similar lines, Norton et al. demonstrated the importance of improving guideline-based GAS ¬testing when they reduced unnecessary testing of patients with viral symptoms from 64% in pre-intervention to 40.5% in the intervention period (14).
With continued research, manufacturers are designing more accurate and sensitive molecular as-says that could eliminate the need for confirmatory culture to detect GAS. Data from a recent study reported a significant increase in positive GAS test results after the laboratory replaced the culture method with Illumigene, a highly sensitive and specific molecular assay (15).
But like other molecular assays, this sensitivity presents its own challenges. Molecular tests can enhance performance in terms of accuracy and TAT. Yet increased detection of GAS either in patients with viral pharyngitis or in GAS carriers could be associated with increased antibiotic prescribing.
Future studies aimed at identifying cutoffs for GAS molecular tests to differentiate infection versus carrier state might be helpful. For example, the molecular platforms that report a cycle threshold (Ct) value for target amplification could be further validated by evaluating the Ct values of PCR with cul-ture-positive and culture-negative specimens. This approach could enable clinical laboratories to set a cutoff value for a true-positive result and disregard the false positives.
Lower and higher Ct values could also help distinguish a corresponding true infection from colonization, respectively, as demonstrated previously for norovirus (16), making molecular assays ideal for GAS testing.
Finally, efforts could also be made to amplify a biomarker specific to bacterial infection in conjunction with GAS to establish its role as a pathogen, either singly or in mixed infection. This approach could be advantageous in the future if multiplex PCRs are designed for pharyngitis testing. Laboratory stewardship efforts aimed at correct patient selection and appropriate method of testing are sure to remain key factors for accurate GAS pharyngitis testing.
Dithi Banerjee, PhD, is a research scientist in the department of pathology and laboratory medicine at Children’s Mercy Hospital in Kansas City, Missouri. Email: dbanerjee[at]cmh.edu
Rangaraj Selvarangan, BVSc, PhD, D(ABMM), FIDSA, is the director of the clinical microbiology, virology and molecular infectious disease laboratory at Children’s Mercy Hospital and a professor in the department of pathology and laboratory medicine at the University of Missouri-School of Medicine in Kansas City, Missouri. Email: rselvarangan[at]cmh.edu
References
- Chiappini E, Regoli M, Bonsignori F, et al. Analysis of different recommendations from inter-national guidelines for the management of acute pharyngitis in adults and children. Clin Ther 2011;33:48–58.
- Zaoutis T, Attia M, Gross R, et al. The role of group C and group G streptococci in acute phar-yngitis in children. Clin Microbiol Infect 2004;10:37–40.
- Lean WL, Arnup S, Danchin M, et al. Rapid diagnostic tests for group A streptococcal pharyn-gitis: A meta-analysis. Pediatrics 2014;134:771–81.
- Fox JW, Cohen DM, Marcon MJ, et al. Performance of rapid streptococcal antigen testing var-ies by personnel. J Clin Microbiol 2006;44:3918–22.
- Ryu SW, Lee JH, Kim J, et al. Comparison of two new generation influenza rapid diagnostic tests with instrument-based digital readout systems for influenza virus detection. Br J Biomed Sci 2016;73:115–20.
- Chapin KC, Blake P, Wilson CD. Performance characteristics and utilization of rapid antigen test, DNA probe, and culture for detection of group a streptococci in an acute care clinic. J Clin Microbiol 2002;40:4207–10.
- Felsenstein S, Faddoul D, Sposto R, et al. Molecular and clinical diagnosis of group A strep-tococcal pharyngitis in children. J Clin Microbiol 2014;52:3884–9.
- Uphoff TS, Buchan BW, Ledeboer NA, et al. Multicenter evaluation of the Solana Group A Streptococcus Assay: Comparison with culture. J Clin Microbiol 2016;54:2388–90.
- Church DL, Lloyd T, Larios O, et al. Evaluation of simplexa group a strep direct kit compared to Hologic group a streptococcal direct assay for detection of group a streptococcus in throat swabs. J Clin Microbiol 2018;56:e01666–17.
- Boyanton BL, Jr., Darnell EM, Prada AE, et al. Evaluation of the Lyra direct strep assay to de-tect group a streptococcus and group C and G beta-hemolytic streptococcus from pharyngeal specimens. J Clin Microbiol 2016;54:175–7.
- Berry GJ, Miller CR, Prats MM, et al. Comparison of the Alere i Strep A Test and the BD Veritor system in the detection of group a streptococcus and the hypothetical impact of results on antibi-otic utilization. J Clin Microbiol 2018;56:e01310–17.
- Wang F, Tian Y, Valcour Y, et al. Performance evaluation of the Cobas Strep A Assay for point-of-care diagnosis of streptococcal pharyngitis in CLIA-waived primary care settings. Open Forum Infectious Diseases 2015;2(suppl_1):1004.
- Shapiro DJ, Lindgren CE, Neuman MI, et al. Viral features and testing for streptococcal phar-yngitis. Pediatrics 2017;139:e20163403.
- Norton LE, Lee BR, Harte L, et al. Improving guideline-based streptococcal pharyngitis testing: A quality improvement initiative. Pediatrics 2018;142:e20172033.
- Tanz RR, Zheng XT, Carter DM, et al. Caution needed: Molecular diagnosis of pediatric group a streptococcal pharyngitis. J Pediatric Infect Dis Soc 2018.16. Kabue JP, Meader E, Hunter PR, et al. Norovirus prevalence and estimated viral load in symptomatic and asymptomatic children from rural communities of Vhembe district, South Africa. J Clin Virol 2016;84:12–18.